|
|
|
Though “Krazy Glue” or “Super Glue” has the ability to seal small wounds, it is not recommended for this purpose since it contains many substances that should not enter the body through the skin, and may be harmful.
Persons who overdose with cardiac glycosides have a better chance of overall survival if they can survive the first 24 hours after the overdose.
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
In 1886, William Bates reported on the discovery of a substance produced by the adrenal gland that turned out to be epinephrine (adrenaline). In 1904, this drug was first artificially synthesized by Friedrich Stolz.
Before a vaccine is licensed in the USA, the Food and Drug Administration (FDA) reviews it for safety and effectiveness. The CDC then reviews all studies again, as well as the American Academy of Pediatrics and the American Academy of Family Physicians. Every lot of vaccine is tested before administration to the public, and the FDA regularly inspects vaccine manufacturers' facilities.
 This engraving of the Boston Massacre (1770) became the most reprinted depiction of the event, and p
This engraving of the Boston Massacre (1770) became the most reprinted depiction of the event, and p
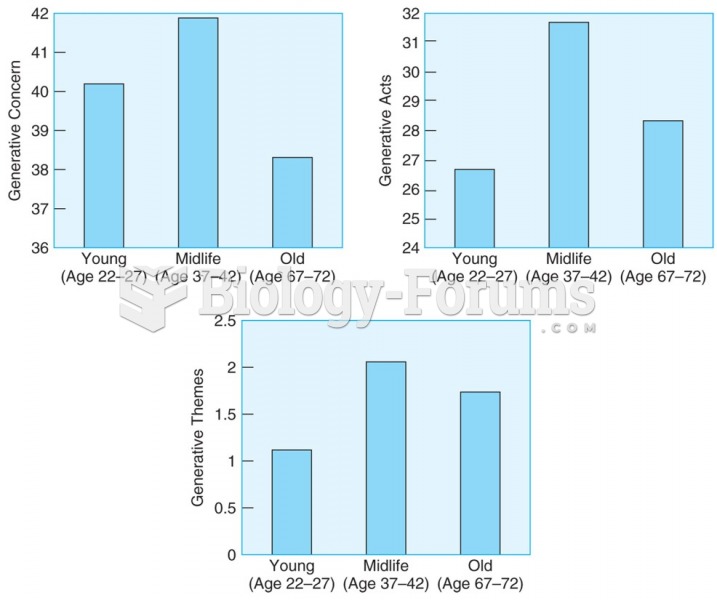 Adults in the middle-aged group (37–42 years) score higher on measures of generativity than either ...
Adults in the middle-aged group (37–42 years) score higher on measures of generativity than either ...