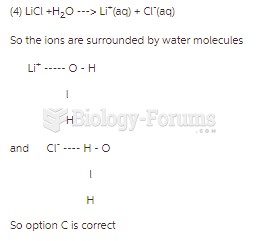
|
|
|
Did you know?
Amoebae are the simplest type of protozoans, and are characterized by a feeding and dividing trophozoite stage that moves by temporary extensions called pseudopodia or false feet.
Did you know?
More than 150,000 Americans killed by cardiovascular disease are younger than the age of 65 years.
Did you know?
According to the CDC, approximately 31.7% of the U.S. population has high low-density lipoprotein (LDL) or "bad cholesterol" levels.
Did you know?
A seasonal flu vaccine is the best way to reduce the chances you will get seasonal influenza and spread it to others.
Did you know?
You should not take more than 1,000 mg of vitamin E per day. Doses above this amount increase the risk of bleeding problems that can lead to a stroke.