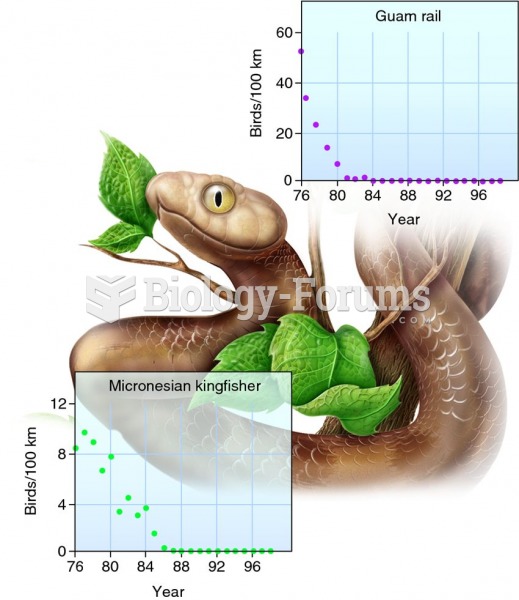
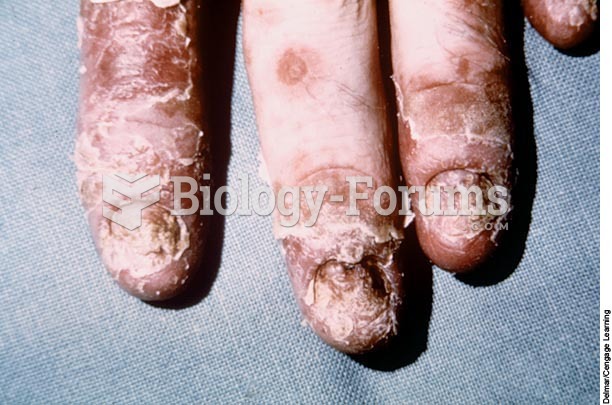
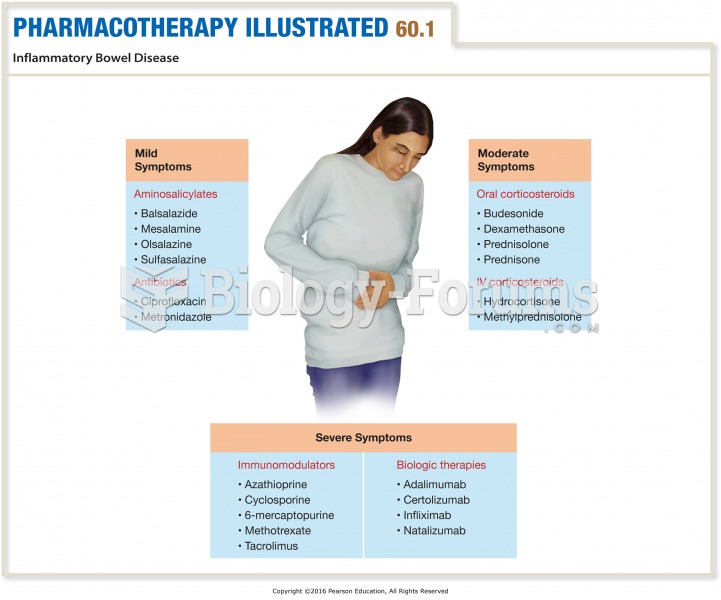
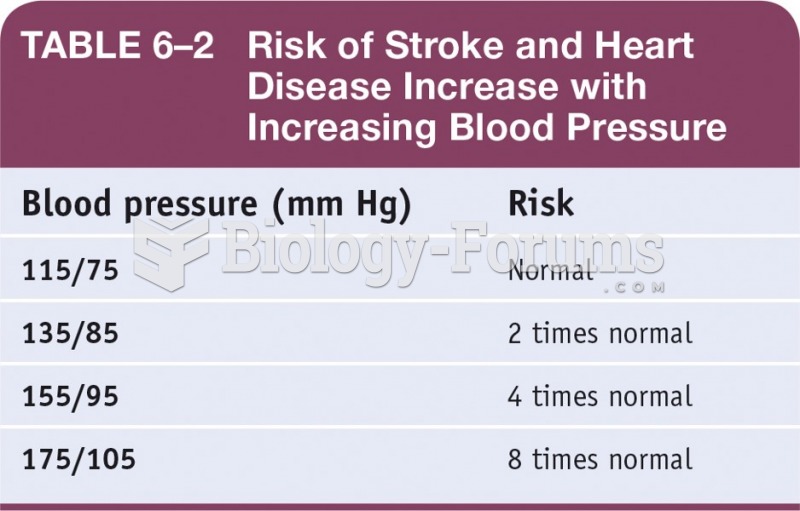
Answer to Question 1
Gall bladder
Answer to Question 2
Although much of its budget comes from Congress, since the 1992 passage of PDUFA (Prescription Drug User Fee Act renewed in 1997, 2002, and 2007, referred to as PDUFA II, III, and IV, respectively), which requires drug companies to pay fees to support the drug review process and companies to pay annual fees for each manufacturing establishment and for each prescription drug product marketed, user fees have steadily risen, until in 1992, 51 percent of the FDA's drug review budget came from the companies that the FDA regulates. Between 1996 and 2006, Congressional appropriations to the FDA stayed the same, whereas from 1998 to 2005, user fees doubled. In 2004, the 232 million in fees made up 53 percent of the FDA's new drug review budget. In 2006, 380 million came from drug user fees.
PDUFA authorized FDA to collect fees from companies that produce certain human drug and biological products. Any time a company wants the FDA to approve a new drug or biologic prior to marketing, it must submit an application along with a fee to support the review process. In addition, companies pay annual fees for each manufacturing establishment and for each prescription drug product marketed. Previously, taxpayers alone paid for product reviews through budgets provided by Congress. In the new program, industry provides the funding in exchange for FDA agreement to meet drug-review performance goals, which emphasize timeliness. In addition to the business funding of FDA, since 1992, Congressional oversight has been sharply curtailed.
PDUFA IV was due to expire in 2007 but was reauthorized by Congress and signed by President Bush on September 27, 2007 . User fees to the FDA were scheduled to rise to 392.8 million, an increase of 87.4 million, by 2008 . However, Sean Hennessy and Brian Strom point out in the New England Journal of Medicine article, PDUFA ReauthorizationDrug Safety's Golden Moment of Opportunity? that a potentially thorny issue is that the large infusion of cash represented by user fees?might result in a conflict of interest for the FDA by creating competing allegiances to pharmaceutical manufacturers and the American People.
PDUFA IV is now set to expire in 2012 . In 2010, the FDA began the process of holding hearings to reauthorize PDUFA. PDUFA V will be presented to Congress in 2012 . Among the areas that the FDA is interested in discussing are ensuring quality of patient-reported outcomes and other endpoint assessment tools and expanding the capacity for scientific advice to address complex manufacturing issues. They appear to be ready to allow more consumer input in their decisions. The FDA is in conflict with pharmaceutical companies over the FDA's proposal to lengthen review times because of the 2007 Congressional drug safety requirements.
A consumer representative (the National Consumer League NCL) stated that the FDA should be funded out of the general treasury. However, it conceded that this would not be possible under current budget conditions. Congress has allowed the regulated industries to dictate how the FDA allocates it resources. NCL believes there is too much at stake to allow this funding model. NCL advocates that the user fees be added to FDA's budget, with no conditions. However, the only financial issue that the FDA discussed was an inflation adjuster.