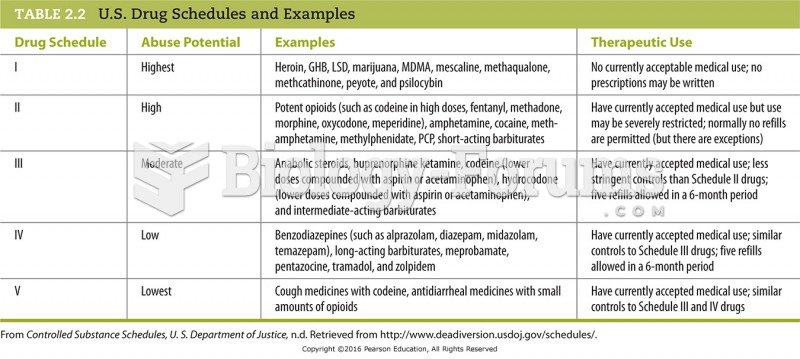
A generic drug is a medication that is chemically equivalent to the brandname drug for which it is a substitute. It is also a bioequivalent. Generic drugs must deliver the same amount of active ingredients into the patient 's bloodstream in the same amount of time, have the same therapeutic action of the brand-name drug, and must be approved by the FDA. Generic drugs in general are less expensive, mostly because manufacturers of generic drugs do not have to repeat the extensive clinical trials that were used in developing the brand-name drug. Though the active ingredients are the same, the fillers and binders used in generic drugs usually differ from those in brand-name drugs, which can affect how quickly they are absorbed or can make them less potent. Some people are allergic to the binders and fillers used in the generic substitute. When a drug is first placed on the market, usually it is patented for 17 years. That means no one can manufacture another drug with the same chemical formulation during those years. Only when the patent has expired can other pharmaceutical companies manufacture generic versions of brand-name drugs. For that reason, some drugs currently on the market do not have generic versions. Certain generic medications may not work as well as the brand-name originals. Doctors generally advise patients to stick with brand names for drugs to control epilepsy, other seizure conditions, heart problems, and psychiatric conditions. What is the relationship between these sentences from the third paragraph? Only when the patent has expired can other pharmaceutical companies manufacture generic versions of brand-name drugs. For that reason, some drugs currently on the market do not have generic versions.
a. example
b. cause and effect
c. statement and clarification
d. summary
Question 2
A generic drug is a medication that is chemically equivalent to the brandname drug for which it is a substitute. It is also a bioequivalent. Generic drugs must deliver the same amount of active ingredients into the patient 's bloodstream in the same amount of time, have the same therapeutic action of the brand-name drug, and must be approved by the FDA. Generic drugs in general are less expensive, mostly because manufacturers of generic drugs do not have to repeat the extensive clinical trials that were used in developing the brand-name drug. Though the active ingredients are the same, the fillers and binders used in generic drugs usually differ from those in brand-name drugs, which can affect how quickly they are absorbed or can make them less potent. Some people are allergic to the binders and fillers used in the generic substitute. When a drug is first placed on the market, usually it is patented for 17 years. That means no one can manufacture another drug with the same chemical formulation during those years. Only when the patent has expired can other pharmaceutical companies manufacture generic versions of brand-name drugs. For that reason, some drugs currently on the market do not have generic versions. Certain generic medications may not work as well as the brand-name originals. Doctors generally advise patients to stick with brand names for drugs to control epilepsy, other seizure conditions, heart problems, and psychiatric conditions. A conclusion that can be drawn from this passage is that
a. all major drugs have generic versions.
b. generic drugs must go through rigorous testing before they can be sold. c. most people are afraid to use generic drugs.
d. doctors prefer for patients to use brand-name drugs.