|
|
|
Did you know?
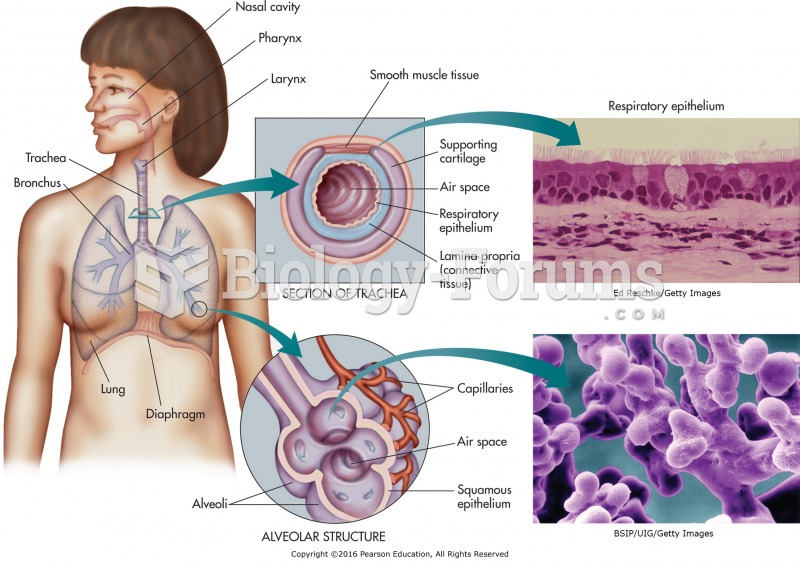
Blood is approximately twice as thick as water because of the cells and other components found in it.
Did you know?
Oliver Wendell Holmes is credited with introducing the words "anesthesia" and "anesthetic" into the English language in 1846.
Did you know?
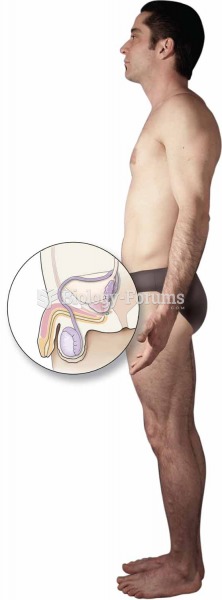
In 1844, Charles Goodyear obtained the first patent for a rubber condom.
Did you know?
Street names for barbiturates include reds, red devils, yellow jackets, blue heavens, Christmas trees, and rainbows. They are commonly referred to as downers.
Did you know?
Illicit drug use costs the United States approximately $181 billion every year.