|
|
|
For about 100 years, scientists thought that peptic ulcers were caused by stress, spicy food, and alcohol. Later, researchers added stomach acid to the list of causes and began treating ulcers with antacids. Now it is known that peptic ulcers are predominantly caused by Helicobacter pylori, a spiral-shaped bacterium that normally exist in the stomach.
Many people have small pouches in their colons that bulge outward through weak spots. Each pouch is called a diverticulum. About 10% of Americans older than age 40 years have diverticulosis, which, when the pouches become infected or inflamed, is called diverticulitis. The main cause of diverticular disease is a low-fiber diet.
The U.S. Preventive Services Task Force recommends that all women age 65 years of age or older should be screened with bone densitometry.
Everyone has one nostril that is larger than the other.
Medications that are definitely not safe to take when breastfeeding include radioactive drugs, antimetabolites, some cancer (chemotherapy) agents, bromocriptine, ergotamine, methotrexate, and cyclosporine.
 Material safety data sheets (MSDS), now called safety data sheets (SDS), should be readily available ...
Material safety data sheets (MSDS), now called safety data sheets (SDS), should be readily available ...
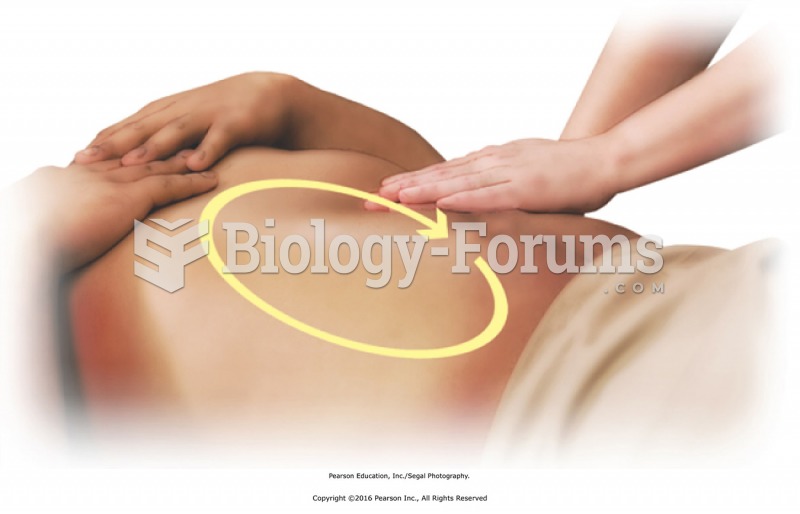 Circular clockwise pattern. Gently lay a hand on the abdomen to establish contact. Apply effleurage ...
Circular clockwise pattern. Gently lay a hand on the abdomen to establish contact. Apply effleurage ...