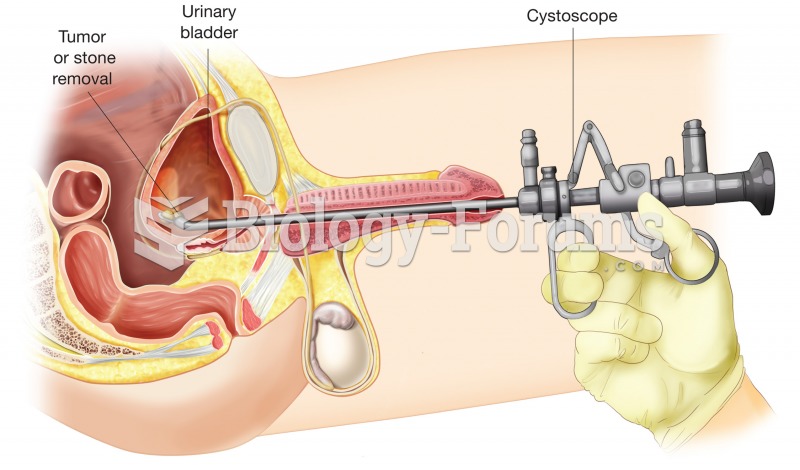
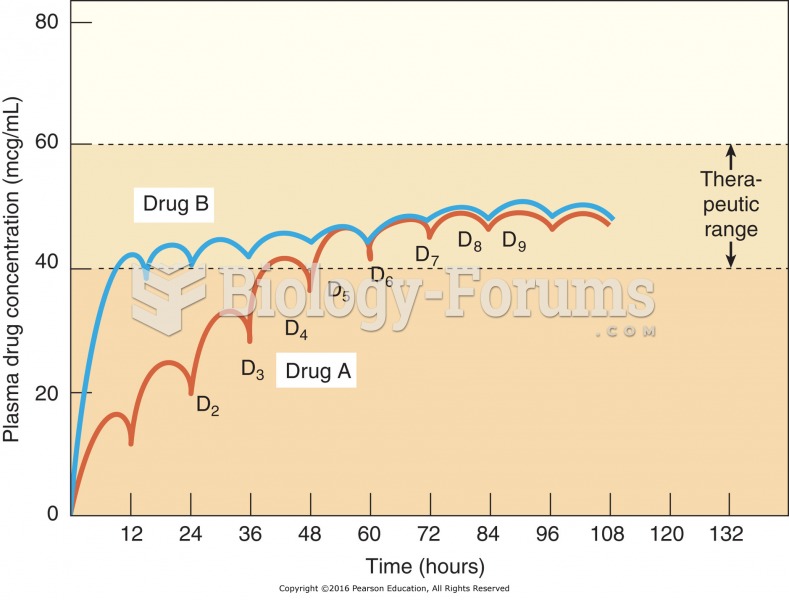
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Today, nearly 8 out of 10 pregnant women living with HIV (about 1.1 million), receive antiretrovirals.
Did you know?
Asthma is the most common chronic childhood disease in the world. Most children who develop asthma have symptoms before they are 5 years old.
Did you know?
The familiar sounds of your heart are made by the heart's valves as they open and close.
Did you know?
The average office desk has 400 times more bacteria on it than a toilet.
Did you know?
Alcohol acts as a diuretic. Eight ounces of water is needed to metabolize just 1 ounce of alcohol.