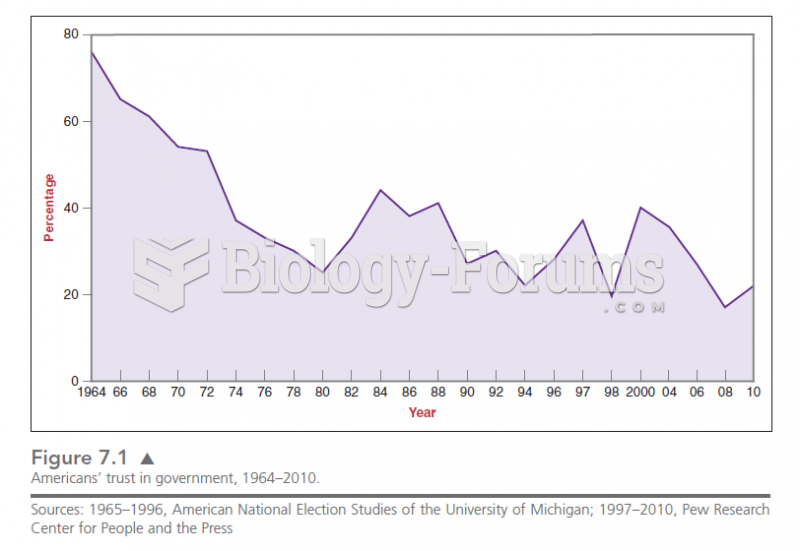
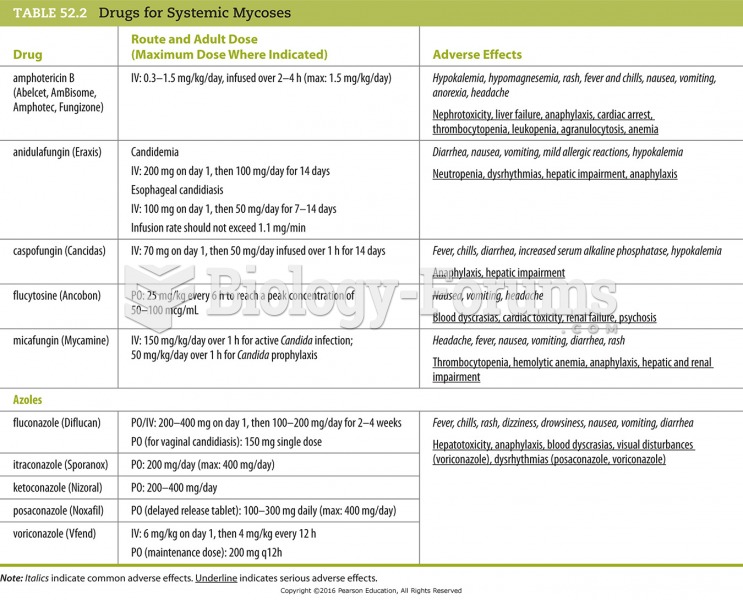
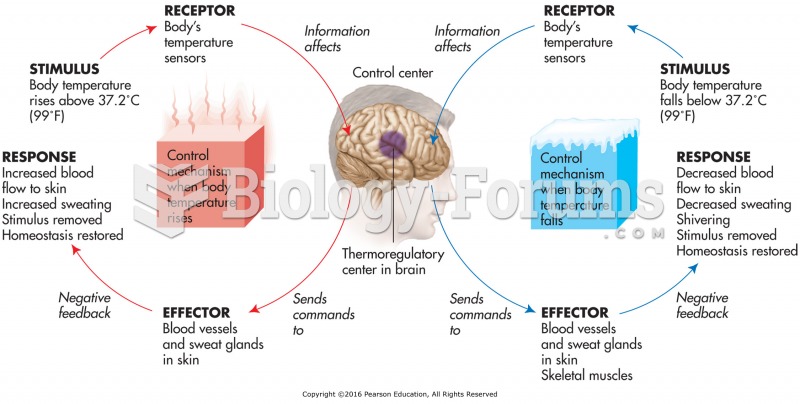
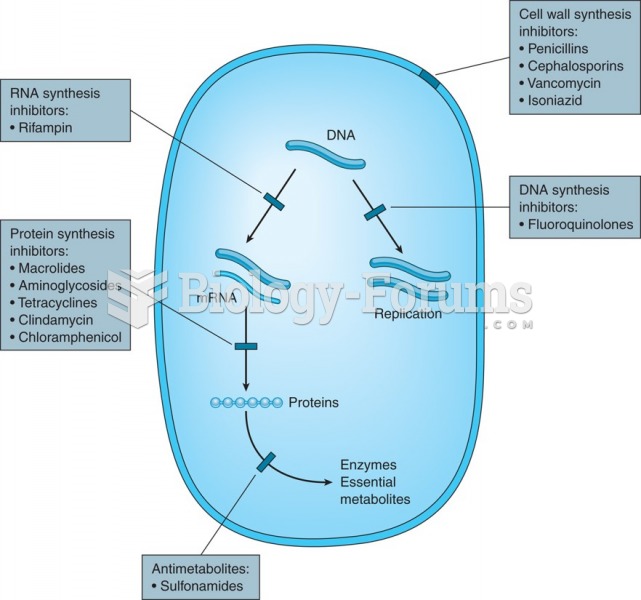
Answer to Question 1
Correct Answer: 3
Rationale 1: The Center for Biologics Evaluation and Research (CBER) regulates the use of biologics, including serums, vaccines, and products found in the bloodstream.
Rationale 2: The Center for Food Safety and Applied Nutrition (CFSAN) regulates use of herbal products and dietary supplements.
Rationale 3: The Center for Drug Evaluation and Research (CDER) has powerful control over whether prescription drugs and OTC drugs may be used for therapy.
Rationale 4: The National Institutes of Health (NIH) do not have control over prescription or OTC drugs.
Global Rationale: The Center for Drug Evaluation and Research (CDER) has powerful control over whether prescription drugs and OTC drugs may be used for therapy. The Center for Biologics Evaluation and Research (CBER) regulates the use of biologics, including serums, vaccines, and products found in the bloodstream. The Center for Food Safety and Applied Nutrition (CFSAN) regulates use of herbal products and dietary supplements. The National Institutes of Health (NIH) do not have control over prescription or OTC drugs.
Answer to Question 2
Correct Answer: 3
Rationale 1: Herbal and dietary supplements may be marketed without prior approval from the FDA.
Rationale 2: Herbal and dietary supplements do not undergo the same testing that prescription or OTC medications do.
Rationale 3: Herbal products and dietary supplements are regulated by the Dietary Supplement Health and Education Act of 1994. This act does not require the same research for herbal and dietary supplements.
Rationale 4: Herbal supplements may be marketed without prior approval from the FDA, and are not required to be tested in clinical trials.
Global Rationale: Herbal products and dietary supplements are regulated by the Dietary Supplement Health and Education Act of 1994. This act does not require the same research for herbal and dietary supplements. Herbal and dietary supplements may be marketed without prior approval from the FDA. Herbal and dietary supplements do not undergo the same testing that prescription or OTC medications do. Herbal supplements may be marketed without prior approval from the FDA, and are not required to be tested in clinical trials.