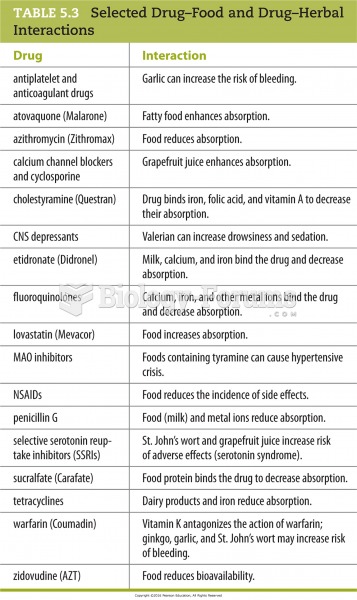
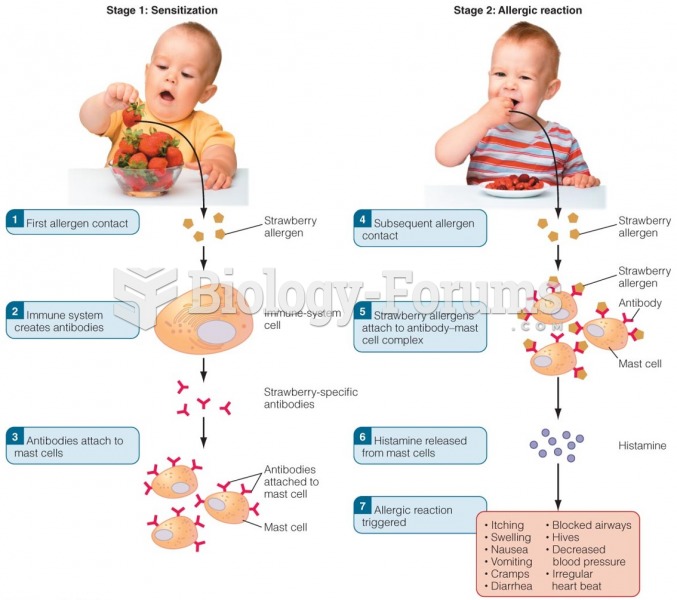
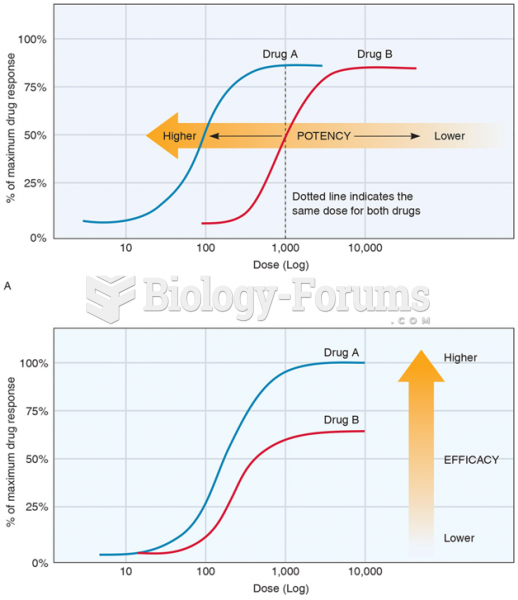
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Egg cells are about the size of a grain of sand. They are formed inside of a female's ovaries before she is even born.
Did you know?
Cucumber slices relieve headaches by tightening blood vessels, reducing blood flow to the area, and relieving pressure.
Did you know?
The average adult has about 21 square feet of skin.
Did you know?
Adolescents often feel clumsy during puberty because during this time of development, their hands and feet grow faster than their arms and legs do. The body is therefore out of proportion. One out of five adolescents actually experiences growing pains during this period.
Did you know?
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.