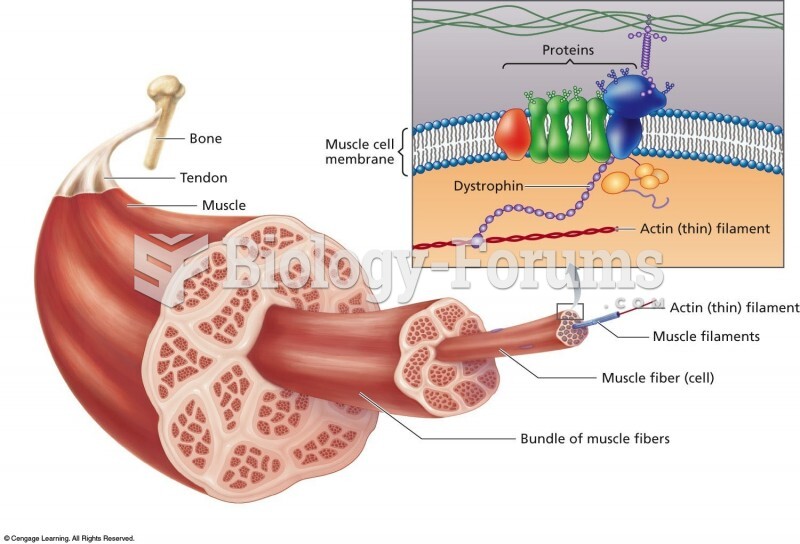
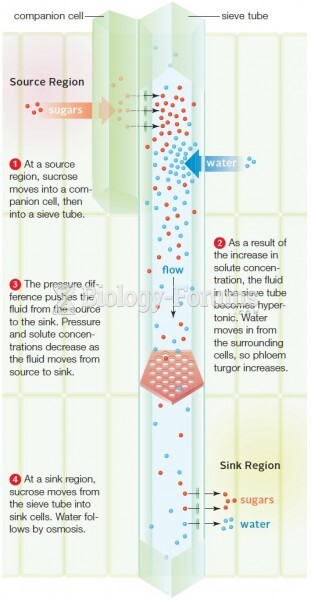
|
|
|
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.
There are 60,000 miles of blood vessels in every adult human.
The eye muscles are the most active muscles in the whole body. The external muscles that move the eyes are the strongest muscles in the human body for the job they have to do. They are 100 times more powerful than they need to be.
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.
The most dangerous mercury compound, dimethyl mercury, is so toxic that even a few microliters spilled on the skin can cause death. Mercury has been shown to accumulate in higher amounts in the following types of fish than other types: swordfish, shark, mackerel, tilefish, crab, and tuna.