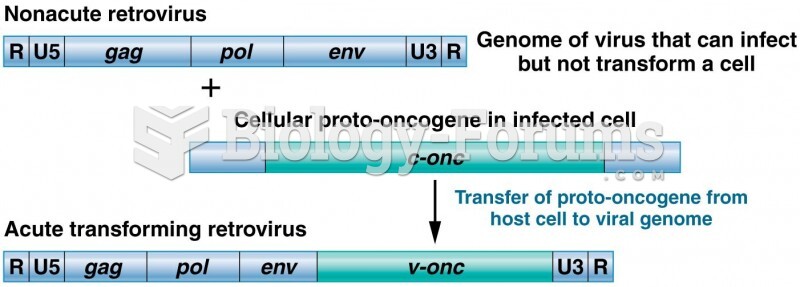
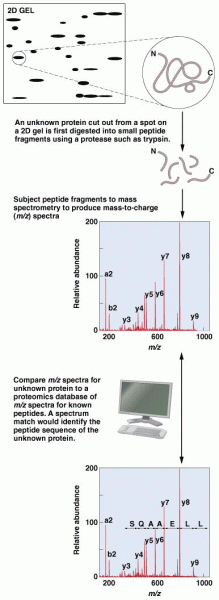
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Essential fatty acids have been shown to be effective against ulcers, asthma, dental cavities, and skin disorders such as acne.
Did you know?
Always store hazardous household chemicals in their original containers out of reach of children. These include bleach, paint, strippers and products containing turpentine, garden chemicals, oven cleaners, fondue fuels, nail polish, and nail polish remover.
Did you know?
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
Did you know?
In 1844, Charles Goodyear obtained the first patent for a rubber condom.
Did you know?
The horizontal fraction bar was introduced by the Arabs.
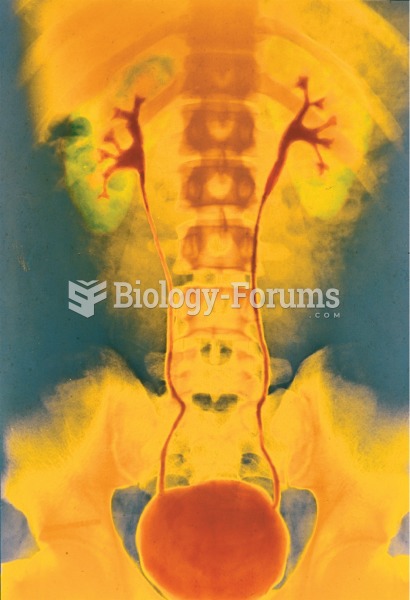 Retrograde pyelogram. A contrast medium is injected into the ureter using a cystoscope, and the X-ra
Retrograde pyelogram. A contrast medium is injected into the ureter using a cystoscope, and the X-ra
 When Union troops pushed toward Richmond in June of 1862, these slaves crossed the Rappahannock Rive
When Union troops pushed toward Richmond in June of 1862, these slaves crossed the Rappahannock Rive