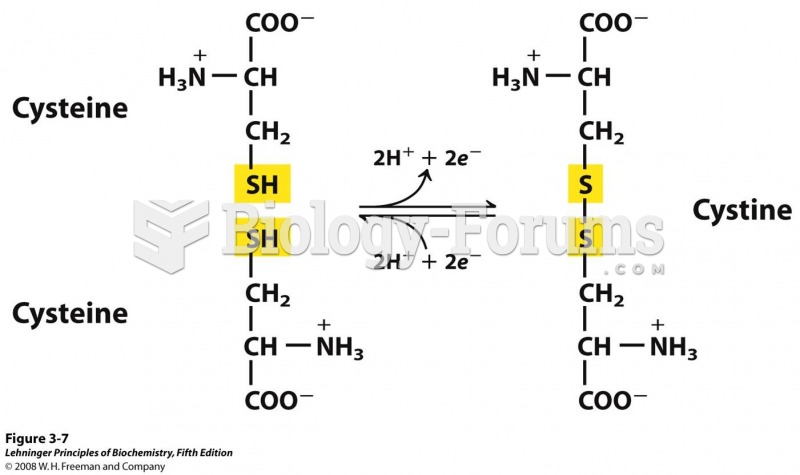
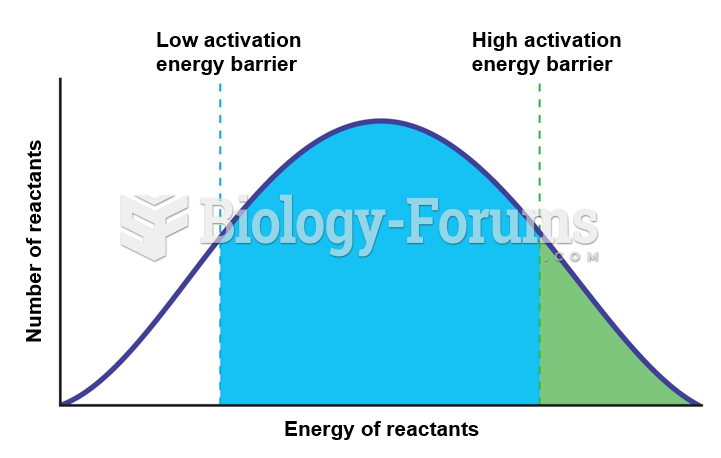
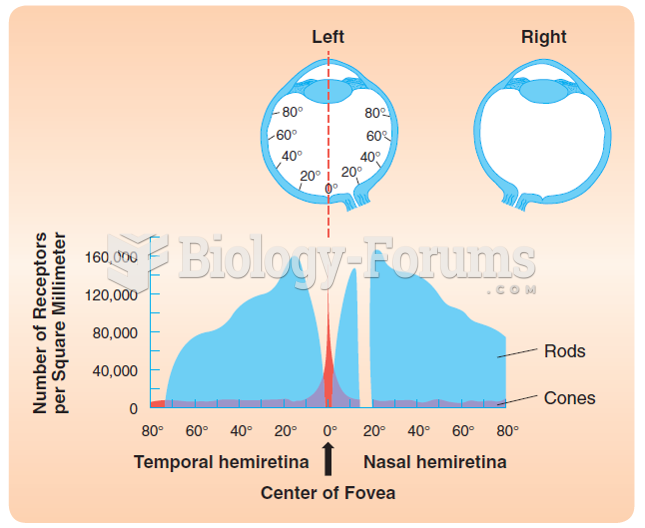
|
|
|
The Babylonians wrote numbers in a system that used 60 as the base value rather than the number 10. They did not have a symbol for "zero."
The average human gut is home to perhaps 500 to 1,000 different species of bacteria.
Human neurons are so small that they require a microscope in order to be seen. However, some neurons can be up to 3 feet long, such as those that extend from the spinal cord to the toes.
The average older adult in the United States takes five prescription drugs per day. Half of these drugs contain a sedative. Alcohol should therefore be avoided by most senior citizens because of the dangerous interactions between alcohol and sedatives.
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.
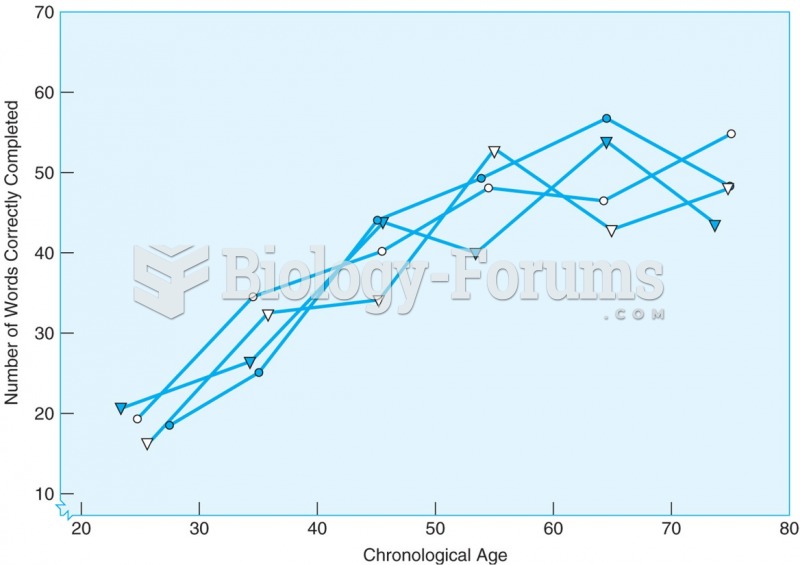 The number of words correctly completed in the New York Times crossword puzzle increases with age ...
The number of words correctly completed in the New York Times crossword puzzle increases with age ...
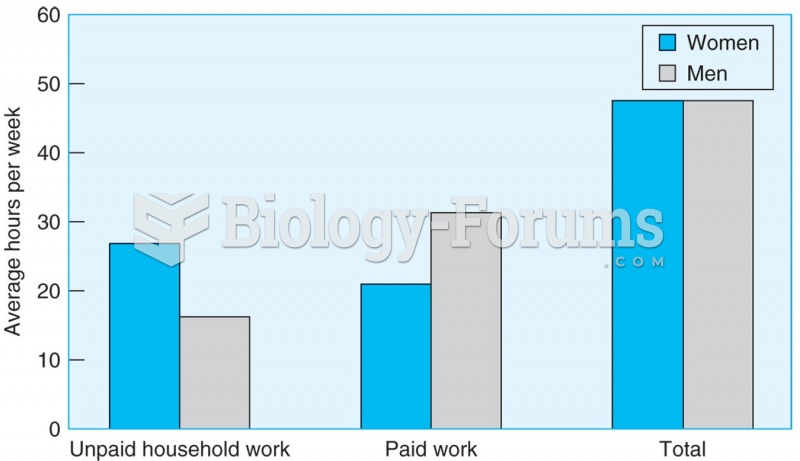 Men and women work the same number of hours a week, but men spend more time on paid work, and women ...
Men and women work the same number of hours a week, but men spend more time on paid work, and women ...