|
|
|
There are more nerve cells in one human brain than there are stars in the Milky Way.
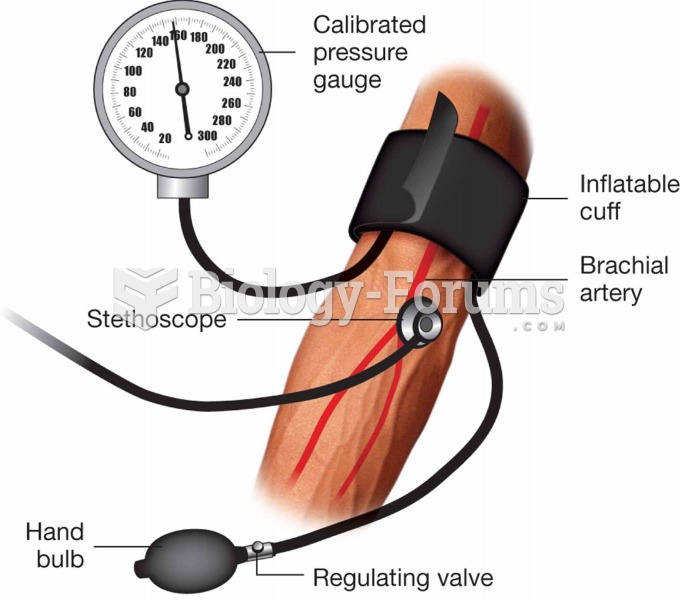
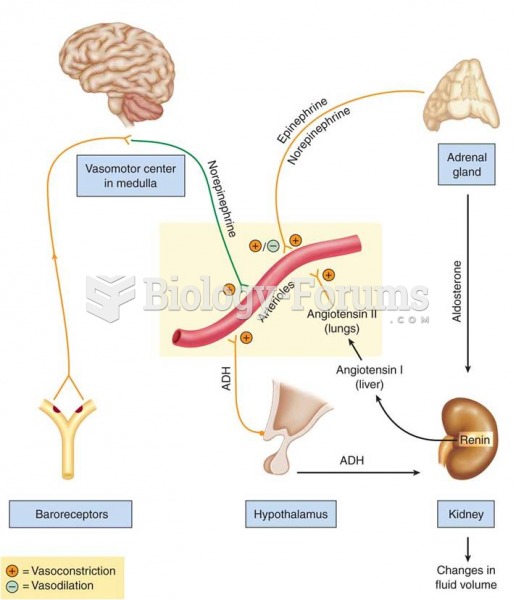
Elderly adults are at greatest risk of stroke and myocardial infarction and have the most to gain from prophylaxis. Patients ages 60 to 80 years with blood pressures above 160/90 mm Hg should benefit from antihypertensive treatment.
Blastomycosis is often misdiagnosed, resulting in tragic outcomes. It is caused by a fungus living in moist soil, in wooded areas of the United States and Canada. If inhaled, the fungus can cause mild breathing problems that may worsen and cause serious illness and even death.
The horizontal fraction bar was introduced by the Arabs.
Human stomach acid is strong enough to dissolve small pieces of metal such as razor blades or staples.