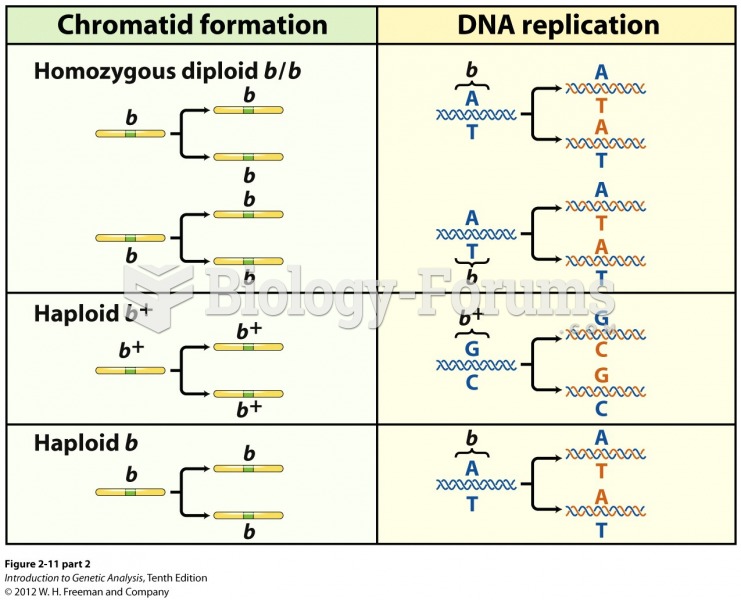
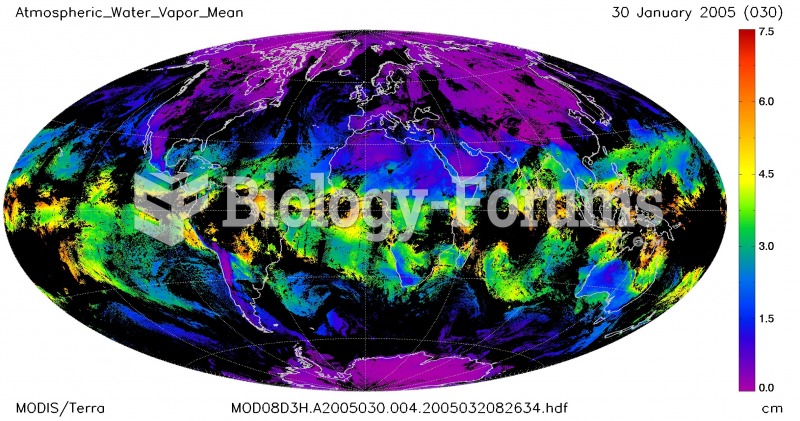
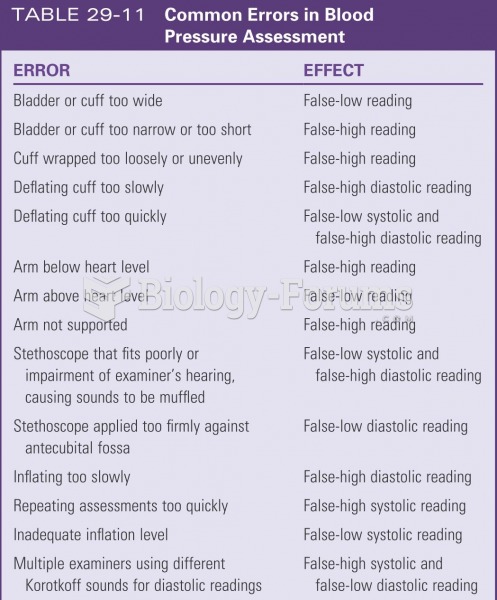
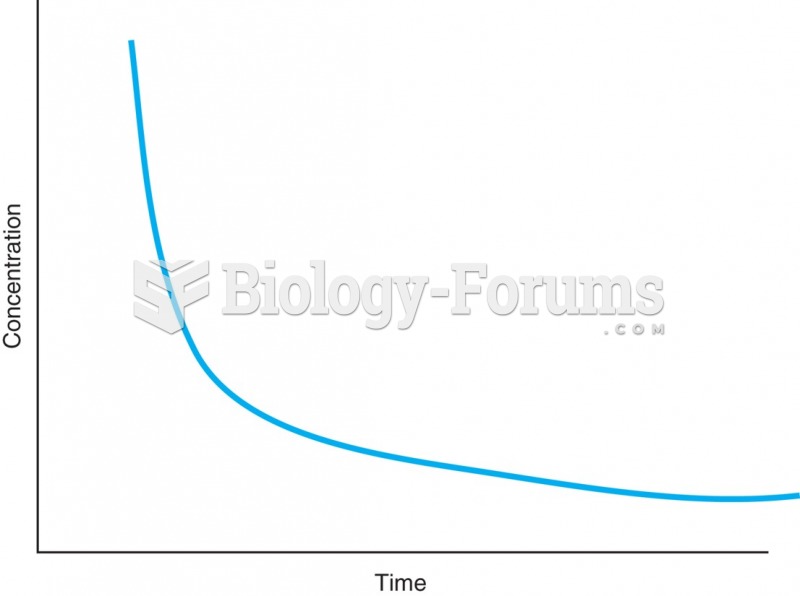
|
|
|
The ratio of hydrogen atoms to oxygen in water (H2O) is 2:1.
It is widely believed that giving a daily oral dose of aspirin to heart attack patients improves their chances of survival because the aspirin blocks the formation of new blood clots.
The lipid bilayer is made of phospholipids. They are arranged in a double layer because one of their ends is attracted to water while the other is repelled by water.
The heart is located in the center of the chest, with part of it tipped slightly so that it taps against the left side of the chest.
In inpatient settings, adverse drug events account for an estimated one in three of all hospital adverse events. They affect approximately 2 million hospital stays every year, and prolong hospital stays by between one and five days.