|
|
|
A serious new warning has been established for pregnant women against taking ACE inhibitors during pregnancy. In the study, the risk of major birth defects in children whose mothers took ACE inhibitors during the first trimester was nearly three times higher than in children whose mothers didn't take ACE inhibitors. Physicians can prescribe alternative medications for pregnant women who have symptoms of high blood pressure.
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.
The most destructive flu epidemic of all times in recorded history occurred in 1918, with approximately 20 million deaths worldwide.
Thyroid conditions may make getting pregnant impossible.
Asthma occurs in one in 11 children and in one in 12 adults. African Americans and Latinos have a higher risk for developing asthma than other groups.
 Species currently endangered in Canada include (a) the Burrowing Owl, Athene cunicularia; (b) Taylor
Species currently endangered in Canada include (a) the Burrowing Owl, Athene cunicularia; (b) Taylor
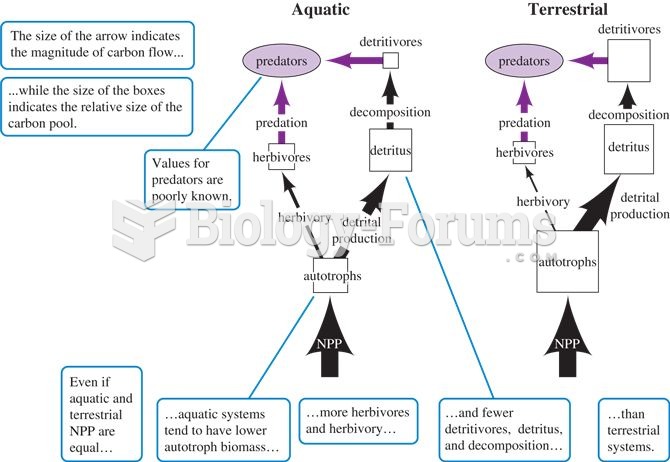 General differences in food webs and carbon flow among terrestrial and aquatic ecosystems (adapted f
General differences in food webs and carbon flow among terrestrial and aquatic ecosystems (adapted f