This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Of the estimated 2 million heroin users in the United States, 600,000–800,000 are considered hardcore addicts. Heroin addiction is considered to be one of the hardest addictions to recover from.
Did you know?
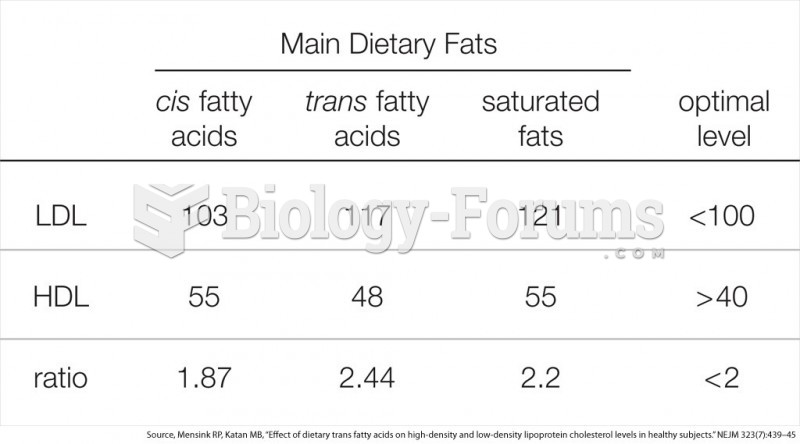
It is widely believed that giving a daily oral dose of aspirin to heart attack patients improves their chances of survival because the aspirin blocks the formation of new blood clots.
Did you know?
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.
Did you know?
Excessive alcohol use costs the country approximately $235 billion every year.
Did you know?
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.