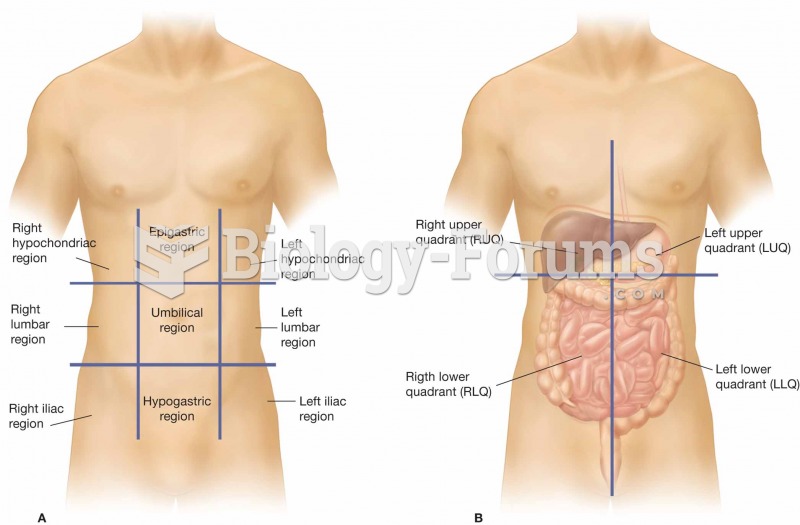
Answer to Question 1
Baking soda, baking powder, steam, air.
Answer to Question 2
Baking soda is also known as Na HCO3 (sodium bicarbonate); baking soda must be used in conjunction with acid to produce flavorful leavening action; acids commonly used include buttermilk, sour milk, lemon juice, vinegar, molasses, or cream of tartar (tartaric acid).
Baking soda requires hydration for leavening. Baking soda is a chemical leavener that is often used in quick bread preparation.
Baking powder is a mixture of sodium bicarbonate (baking soda), dry acid, & starch; baking powder is either single- or double-acting powder. This is determined by how quickly the acid-reacting substances go into solution. Tartrate baking powder is an example of a single-acting baking powder. It contains tartaric acid and potassium acid tartrate, which are both soluble in cold water. Therefore, carbon dioxide will be released in the dough before the dough is heated in the oven. An example of a double-acting baking powder is SAS - phosphate baking powder. The acid-reacting substances are monocalcium phosphate monohydrate, which is soluble in cold water, and sodium aluminum sulfate, which is soluble in hot water. Therefore, only some of the carbon dioxide is produced in the cold dough and the remainder is produced in the dough during baking because the sodium aluminum sulfate does not dissolve and react until the dough is heated. Because the leavening gas is produced in two steps, this is a double-acting baking powder. Most commercial baking powders are double acting. Baking powder is a chemical leavener that is often used in quick bread preparation.
Self-rising flour contains sodium bicarbonate (baking soda), acid reacting substances, and salt and is used in recipes for its leavening ability.
Yeast is a microorganism-based leavener. Sugar serves as a food source for yeast, and it yields ethanol and carbon dioxide. Yeast ferments all simple sugars except lactose. Yeast is used for leavening in yeast bread products and is not used in quick bread recipes.
Answer to Question 3
Acid added prior to gelatinization decreases viscosity of the paste by hydrolyzing the starch to form smaller dextrin molecules. Therefore, starch mixtures should be cooked and gelatinization should occur before adding acid. Acid should be added while the starch paste is hot to avoid disturbance and weakening of the gel.