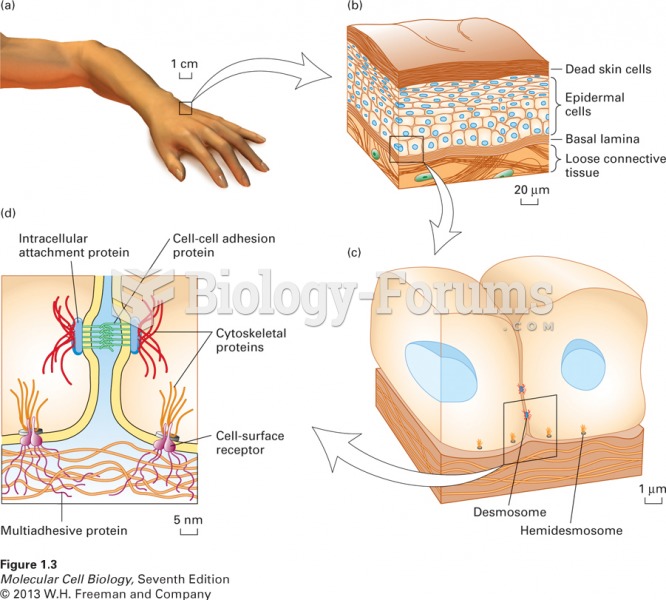
Answer to Question 1
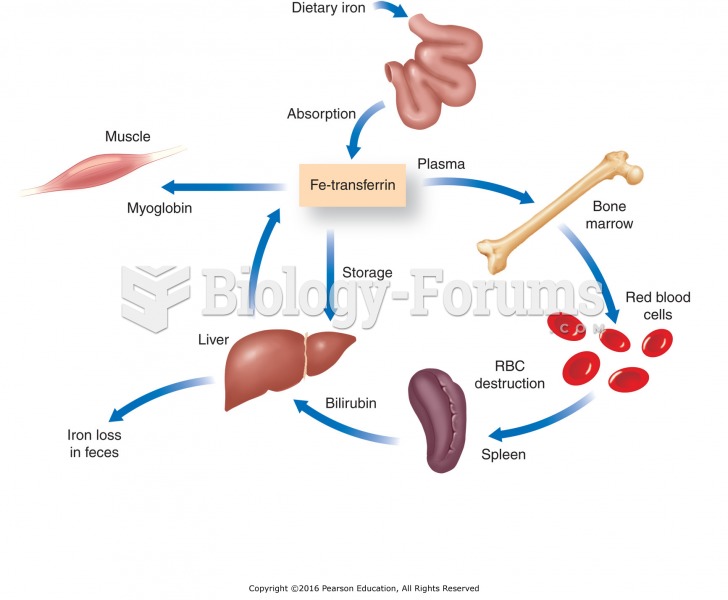
Every living cellboth plant and animalcontains iron. Most of the iron in the body is a component of the proteins hemoglobin in red blood cells and myoglobin in muscle cells. The iron in both hemoglobin and myoglobin helps them carry and hold oxygen and then release it. Hemoglobin in the blood carries oxygen from the lungs to tissues throughout the body. Myoglobin holds oxygen for the muscles to use when they contract. As part of many enzymes, iron is vital to the processes by which cells generate energy. Iron is also needed to make new cells, amino acids, hormones, and neurotransmitters.
The special provisions the body makes for iron's handling show that it is a precious mineral to be tightly hoarded. For example, when a red blood cell dies, the liver saves the iron and returns it to the bone marrow, which uses it to build new red blood cells. The body does lose iron from the digestive tract, in nail and hair trimmings, and in shed skin cells, but only in tiny amounts. Bleeding, however, can cause significant iron loss from the body.
Special measures are needed to maintain an appropriate iron balance in the body. Iron is a powerful oxidant that generates free-radical reactions. Free radicals increase oxidative stress and inflammation associated with diseases such as diabetes, heart disease, and cancer. To ensure that enough iron is available to meet the body's needs and yet guard against excessive and damaging levels, special proteins transport and store the body's iron supply, and its absorption is tightly regulated. Because the body has no active means of excreting excess iron, regulation of absorption plays a critical role in iron homeostasis.
Answer to Question 2
When mineral salts dissolve in water, they separate (dissociate) into charged particles known as ions, which can conduct electricity. For this reason, a salt that dissociates in water is known as an electrolyte. The body fluids, which contain water and partly dissociated salts, are electrolyte solutions.
The body's electrolytes are vital to the life of the cells and therefore must be closely regulated to help maintain the appropriate distribution of body fluids. The major minerals form salts that dissolve in the body fluids; the cells direct where these salts go; and the movement of the salts determines where the fluids flow because water follows salt. Cells use this force, called osmosis, to move fluids back and forth across their membranes. Thanks to the electrolytes, water can be held in compartments where it is needed.
Proteins in the cell membranes move ions into or out of the cells. These protein pumps tend to concentrate sodium and chloride outside cells and potassium and other ions inside. By maintaining specific amounts of sodium outside and potassium inside, cells can regulate the exact amounts of water inside and outside their boundaries.
Healthy kidneys regulate the body's sodium, as well as its water, with remarkable precision. The intestinal tract absorbs sodium readily, and it travels freely in the blood, but the kidneys excrete unneeded amounts. The kidneys actually filter all of the sodium out of the blood; then they return to the bloodstream the exact amount the body needs to retain. Thus, the body's total electrolytes remain constant, while the urinary electrolytes fluctuate according to what is eaten.
In some cases, the body's mechanisms for maintaining fluid and electrolyte balances cannot compensate for a sudden loss of large amounts of fluid and electrolytes. Vomiting, diarrhea, heavy sweating, fever, burns, wounds, and the like may incur great fluid and electrolyte losses, precipitating an emergency that demands medical intervention.