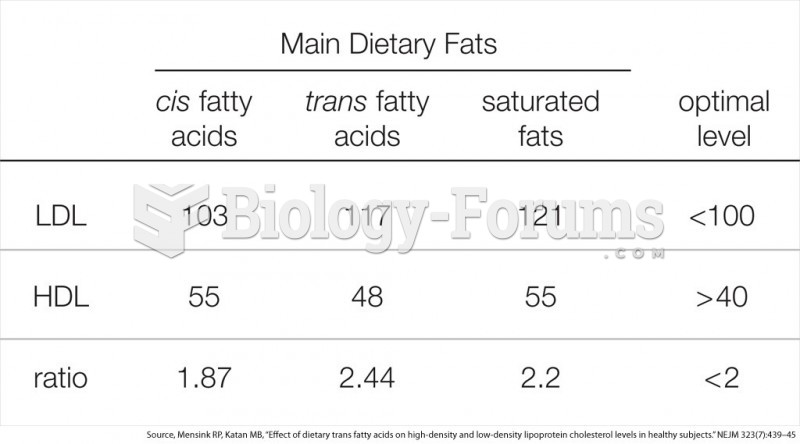
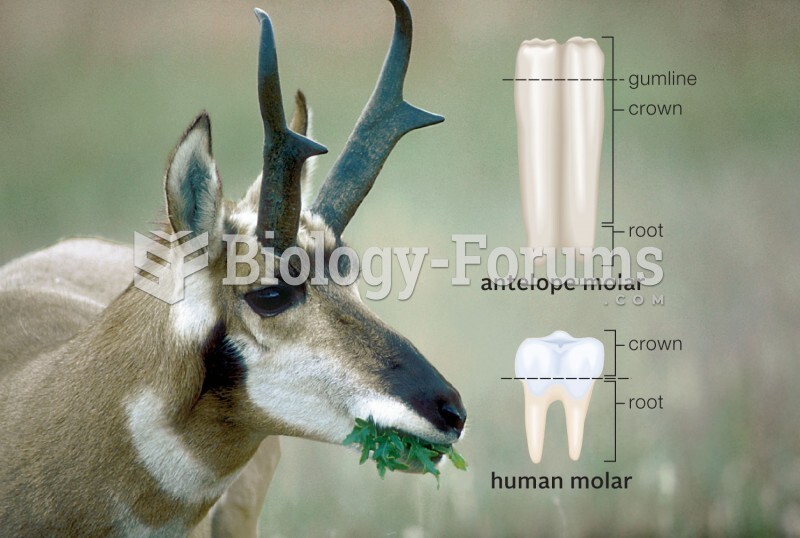
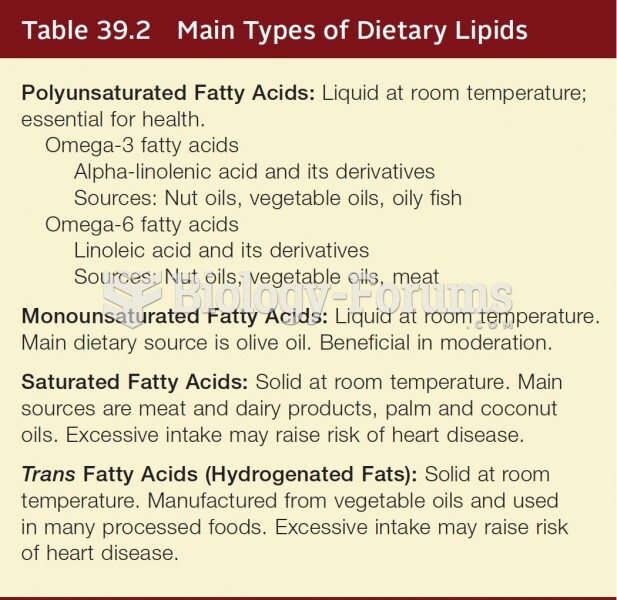
Answer to Question 11 . Undertake research on the supplement you are interested in taking at sites offered by the National Institutes of Health:
http://dsld.nlm.nih.gov and
www.nlm.nih.gov/medlineplus /druginfo/herb_All.html.
2 . Purchase supplements labeled USP or CL. They are tested for purity, ingredients, and dose.
3 . Check the expiration date on supplements. Use unexpired supplements.
4 . Choose vitamin and mineral supplements containing 100 of the Daily Value or less.
5 . Take vitamin and mineral supplements with meals.
6 . If you have a diagnosed need for a specific vitamin or mineral, take that individual vitamin or mineral and not a multiple supplement.
7 . Store supplements where small children cannot get at them.
8 . Don't use herbal remedies for serious, self-diagnosed conditions such as depression, persistent headaches, and memory loss. (You might benefit more from a different treatment.)
9 . Don't use herbal remedies without medical advice if you are attempting to become pregnant or if you are pregnant or breastfeeding.
10 . Don't mix herbal remedies.
11 . If you are allergic to certain plants, make sure herbal remedies are not going to be a problem before you use them.
12 . If you have a bad reaction to an herbal remedy, stop using it and report the reaction to the FDA using the site:
www.fda.gov/Safety/MedWatch/HowToReport/ucm053074.htm.
13 . Tell your health care provider about the dietary supplements you take or plan on taking.
Answer to Question 21 . Product must be labeled Dietary Supplement..
2 . Product must have a Supplemental Facts label that includes serving size, amount of the product per serving, Daily Value of key nutrients of public health significance, a list of other ingredients, and the manufacturer's name and address.
3 . Nutrient claims (such as low in sodium and high in fiber) can be made on labels of products that qualify based on nutrition labeling regulations.
4 . A listing of ingredients in the supplement.
5 . Structure/function claims about how the product affects normal body structures (such as helps maintain strong bones) or functions (enhances normal bowel function) can be made on product labels. If a structure/function claim is made, this FDA disclaimer must appear: This statement has not been evaluated by the FDA. This product is not intended to diagnose, treat, cure, or prevent any disease.