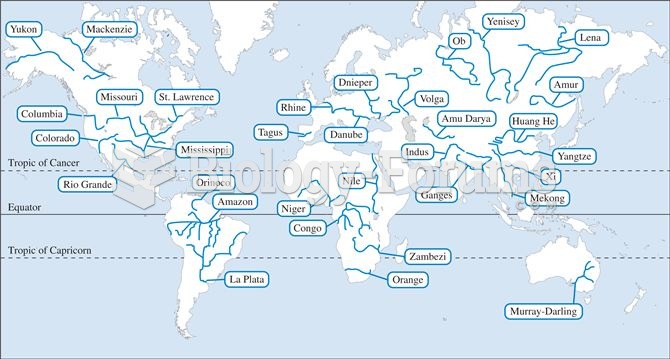
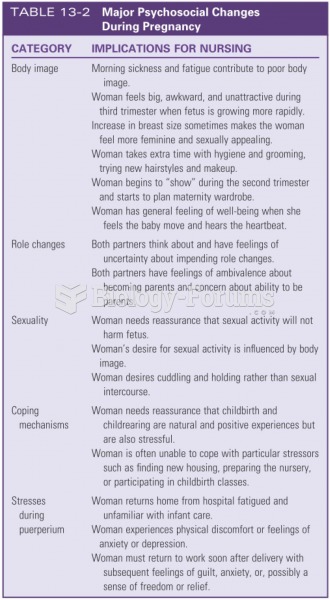
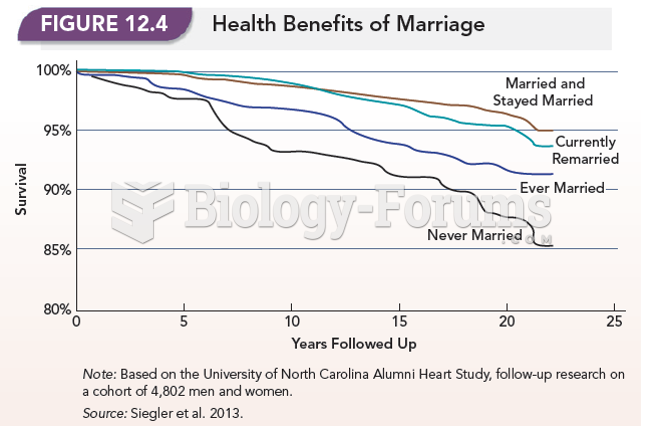
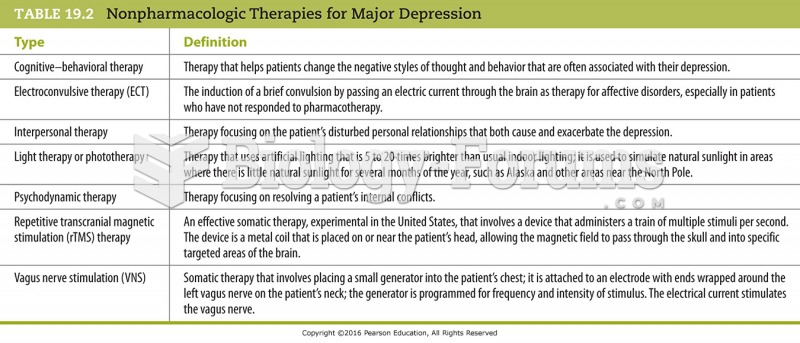
Answer to Question 1
Details of supplement regulation are defined in the Dietary Supplement Health and Education Act of 1994, which was intended to enable consumers to make informed choices about dietary supplements. The act subjects supplements to the same general labeling requirements that apply to foods. Specifically:
Nutrition labeling for dietary supplements is required.
Labels may make nutrient claims (as high or low) according to specific criteria (for example, an excellent source of vitamin C).
Labels may claim that the lack of a nutrient can cause a deficiency disease, but if they do, they must also include the prevalence of that deficiency disease in the United States.
Labels may make health claims that are supported by significant scientific agreement and are not brand specific (for example, folate protects against neural tube defects).
Labels may claim to diagnose, treat, cure, or relieve common complaints such as menstrual cramps or memory loss, but may not make claims about specific diseases (except as noted previously).
Labels may make structure-function claims about the role a nutrient plays in the body, how the nutrient performs its function, and how consuming the nutrient is associated with general well-being. The manufacturer is responsible for ensuring that the claims are truthful and not misleading. Claims must be accompanied by an FDA disclaimer statement: This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
Answer to Question 2
Supplements with a USP verification logo have been tested by the US Pharmacopeia (USP) to ensure that the supplement:
Contains the declared ingredients and amounts listed on the label
Does not contain harmful levels of contaminants
Will disintegrate and release ingredients in the body
Was made under safe and sanitary conditions