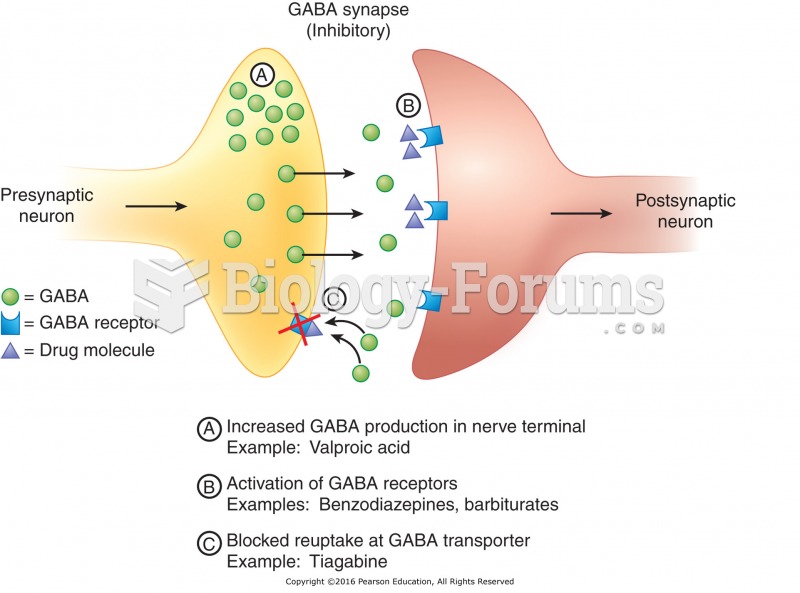
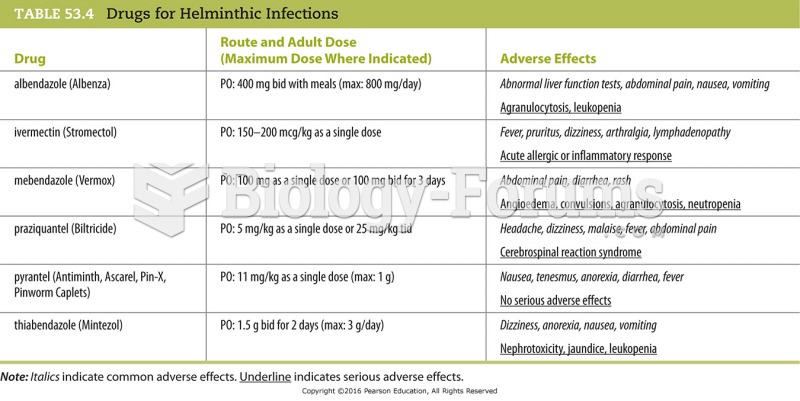
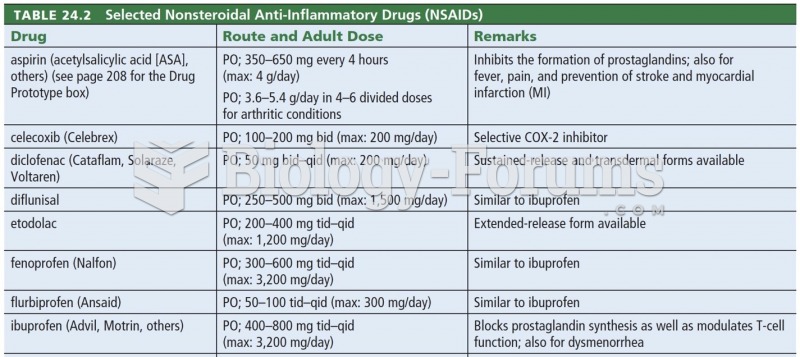
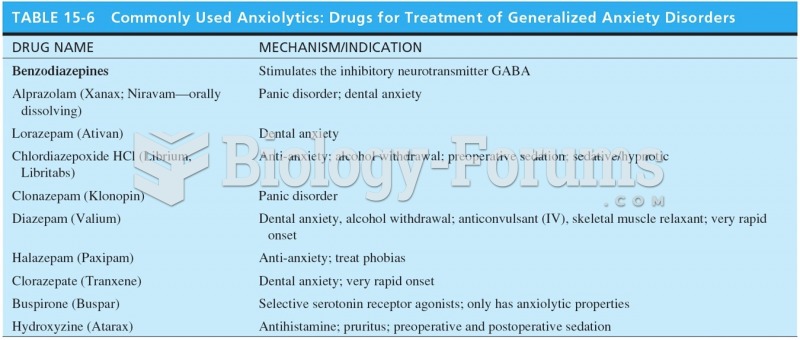
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.
Did you know?
Never take aspirin without food because it is likely to irritate your stomach. Never give aspirin to children under age 12. Overdoses of aspirin have the potential to cause deafness.
Did you know?
Women are 50% to 75% more likely than men to experience an adverse drug reaction.
Did you know?
Atropine was named after the Greek goddess Atropos, the oldest and ugliest of the three sisters known as the Fates, who controlled the destiny of men.
Did you know?
Atropine, along with scopolamine and hyoscyamine, is found in the Datura stramonium plant, which gives hallucinogenic effects and is also known as locoweed.