|
|
|
Normal urine is sterile. It contains fluids, salts, and waste products. It is free of bacteria, viruses, and fungi.
Adult head lice are gray, about ? inch long, and often have a tiny dot on their backs. A female can lay between 50 and 150 eggs within the several weeks that she is alive. They feed on human blood.
Anesthesia awareness is a potentially disturbing adverse effect wherein patients who have been paralyzed with muscle relaxants may awaken. They may be aware of their surroundings but unable to communicate or move. Neurologic monitoring equipment that helps to more closely check the patient's anesthesia stages is now available to avoid the occurrence of anesthesia awareness.
Adults are resistant to the bacterium that causes Botulism. These bacteria thrive in honey – therefore, honey should never be given to infants since their immune systems are not yet resistant.
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
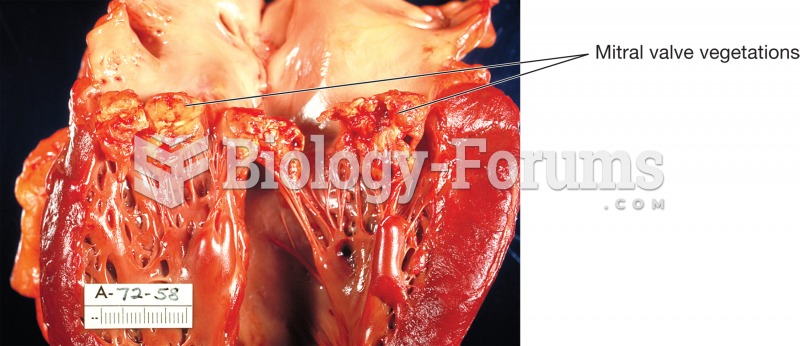 Endocarditis. The human heart has been sectioned to reveal the left ventricle and origin of the aort
Endocarditis. The human heart has been sectioned to reveal the left ventricle and origin of the aort
 In 1839 Mary Cragin, at the age of twenty-nine, became a convert to John Humphrey Noyes’s “communism
In 1839 Mary Cragin, at the age of twenty-nine, became a convert to John Humphrey Noyes’s “communism





