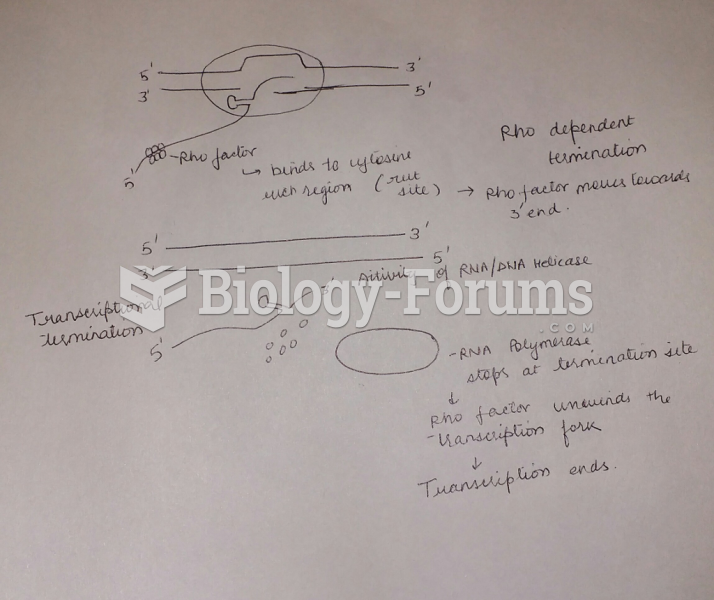
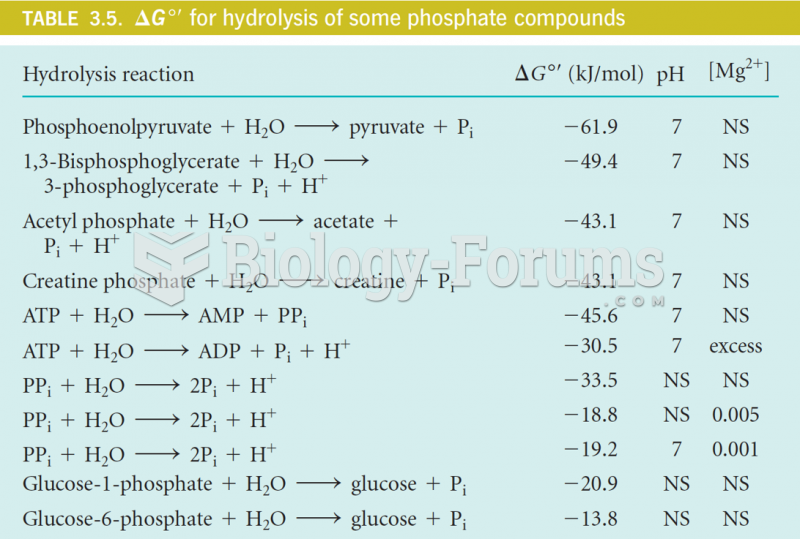
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.
Did you know?
The most destructive flu epidemic of all times in recorded history occurred in 1918, with approximately 20 million deaths worldwide.
Did you know?
The familiar sounds of your heart are made by the heart's valves as they open and close.
Did you know?
The lipid bilayer is made of phospholipids. They are arranged in a double layer because one of their ends is attracted to water while the other is repelled by water.
Did you know?
In inpatient settings, adverse drug events account for an estimated one in three of all hospital adverse events. They affect approximately 2 million hospital stays every year, and prolong hospital stays by between one and five days.
 Historian James Merrell notes several errors in Benjamin West’s famous 1771 painting, William Penn’s
Historian James Merrell notes several errors in Benjamin West’s famous 1771 painting, William Penn’s
 Headsets are ergonomically correct and allow the medical assistant or receptionist to use both hands
Headsets are ergonomically correct and allow the medical assistant or receptionist to use both hands