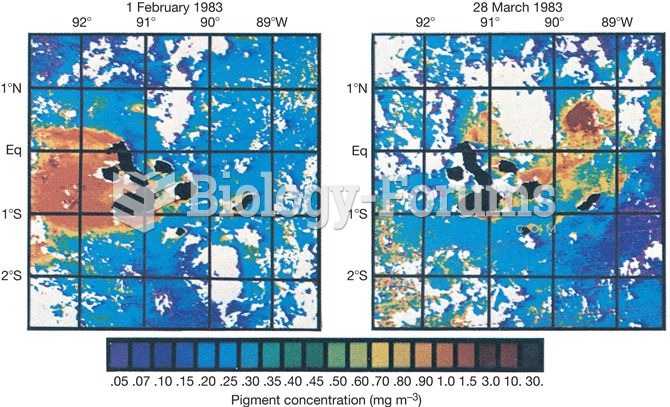
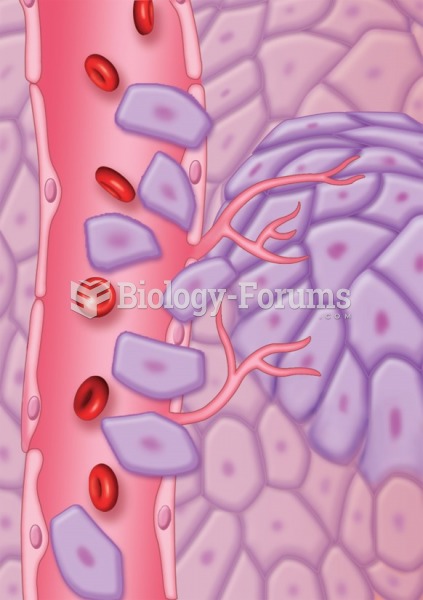
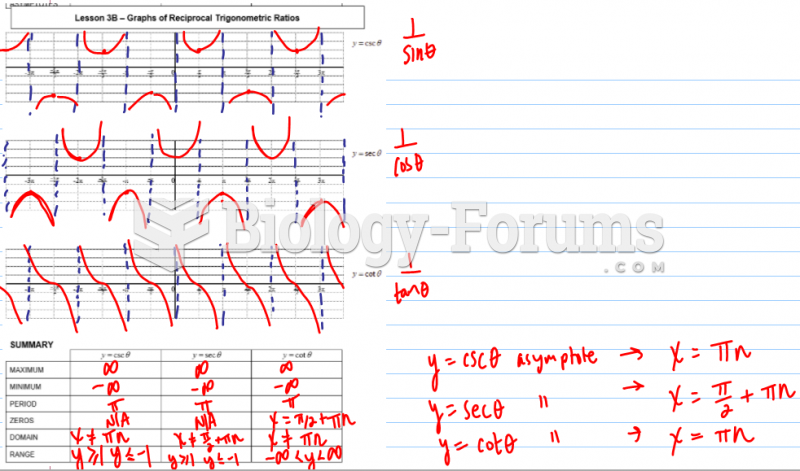
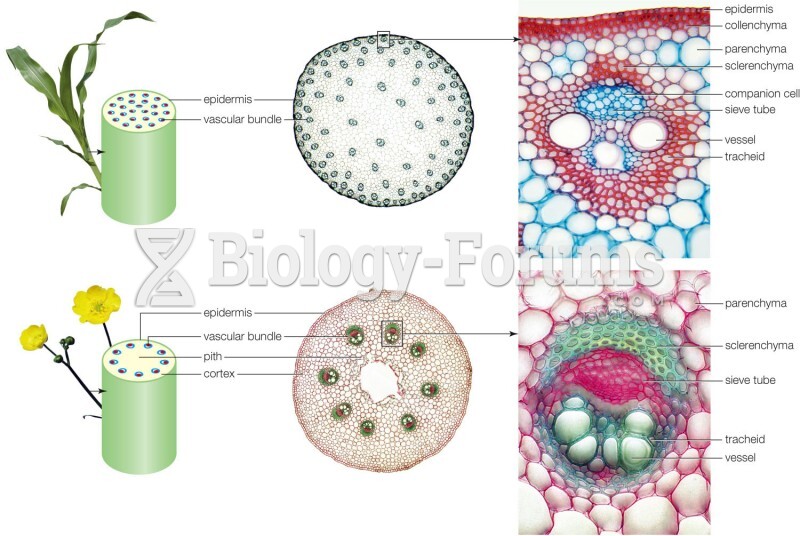
Which of the following compounds are primary (1) amines?

a.
1 and 2
b.
1 and 3
c.
1, 2 and 3
d.
1, 2, 3 and 4
Question 2Instructions: Give the major organic product(s) of each of the following reaction or sequence of reactions. Show all relevant stereochemistry.
Give the major product(s).
 Question 3
Question 3Instructions: Give the major organic product(s) of each of the following reaction or sequence of reactions. Show all relevant stereochemistry.
Give major product(s):
 Question 4
Question 4Instructions: Give the major organic product(s) of each of the following reaction or sequence of reactions. Show all relevant stereochemistry.
 Question 5
Question 5Instructions: Give the major organic product(s) of each of the following reaction or sequence of reactions. Show all relevant stereochemistry.
Give major product(s):
 Question 6
Question 6Instructions: Give the major organic product(s) of each of the following reaction or sequence of reactions. Show all relevant stereochemistry.
Give major product(s):
 Question 7
Question 7Rank the following compounds in order of increasing basicity. Label the least basic compound 1 and the most basic compound 4. Place the number corresponding to the compound's rank in the blank below the compound.
 Question 8
Question 8In the following series of compounds, which is the most basic and the least basic? Explain your choices.
 Question 9
Question 9Circle any of the following that would be classified as a heterocyclic amine.
 Question 10
Question 10Instructions: Consider the reaction below to answer the following question(s).
Methamphetamine can be synthesized by reacting phenyl-2-propanone with methylamine in the presence of H
2/Ni.

Refer to instructions. Although the yield of methamphetamine is good, some unreacted phenyl-2-propanone remains after the reaction is complete. Describe how methamphetamine can be separated from phenyl-2-propanone.
Question 11Instructions: Consider the reaction below to answer the following question(s).
Methamphetamine can be synthesized by reacting phenyl-2-propanone with methylamine in the presence of H
2/Ni.

Refer to instructions. The synthesis of methamphetamine by reaction of 2-phenyl-2 propanone with methylamine in the presence of H
2/Ni is referred to as:
a.
reductive nitrogenation
b.
reactive nitrogenation
c.
reductive amination
d.
reactive amination
Question 12Instructions: Consider the reaction below to answer the following question(s).
Methamphetamine can be synthesized by reacting phenyl-2-propanone with methylamine in the presence of H
2/Ni.

Refer to instructions. Intermediate A is an example of:
a.
an imine
b.
an enamine
c.
an iminium ion
d.
an imide
Question 13Instructions: Consider the reaction below to answer the following question(s).
Methamphetamine can be synthesized by reacting phenyl-2-propanone with methylamine in the presence of H
2/Ni.

Refer to instructions. Identify the nucleophile in the initial reaction of phenyl-2-propanone to yield intermediate A and classify the type of reaction that produces the final product from A.
Question 14Instructions: Based on the following structures, name the compound.
Name:
 Question 15
Question 15Instructions: Based on the following structures, name the compound.
Name:
 Question 16
Question 16Instructions: Based on the following structures, name the compound.
Name:
 Question 17
Question 17Instructions: Based on the following structures, name the compound.
Name:
 Question 18
Question 18Instructions: Draw structures corresponding to each of the IUPAC names given in the following question(s).
Draw:
N,N-dimethylcyclopentylamine
Question 19Instructions: Draw structures corresponding to each of the IUPAC names given in the following question(s).
Draw:
hexane-1,6-diamine