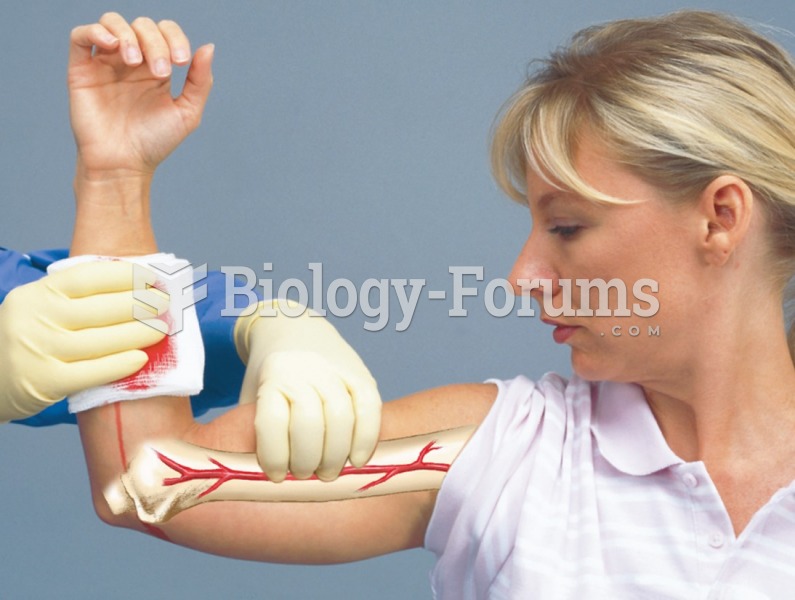
|
|
|
GI conditions that will keep you out of the U.S. armed services include ulcers, varices, fistulas, esophagitis, gastritis, congenital abnormalities, inflammatory bowel disease, enteritis, colitis, proctitis, duodenal diverticula, malabsorption syndromes, hepatitis, cirrhosis, cysts, abscesses, pancreatitis, polyps, certain hemorrhoids, splenomegaly, hernias, recent abdominal surgery, GI bypass or stomach stapling, and artificial GI openings.
Malaria mortality rates are falling. Increased malaria prevention and control measures have greatly improved these rates. Since 2000, malaria mortality rates have fallen globally by 60% among all age groups, and by 65% among children under age 5.
Approximately 500,000 babies are born each year in the United States to teenage mothers.
Elderly adults are living longer, and causes of death are shifting. At the same time, autopsy rates are at or near their lowest in history.
Long-term mental and physical effects from substance abuse include: paranoia, psychosis, immune deficiencies, and organ damage.
 The air pressure going to the nozzle should be reduced to 30 psi or less to help prevent personal ...
The air pressure going to the nozzle should be reduced to 30 psi or less to help prevent personal ...
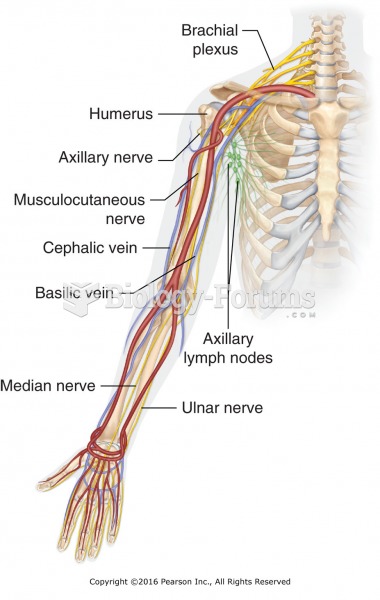 The anterior arm (shoulder, axilla, anticubital, and inner wrist areas). Approach deep pressure in ...
The anterior arm (shoulder, axilla, anticubital, and inner wrist areas). Approach deep pressure in ...
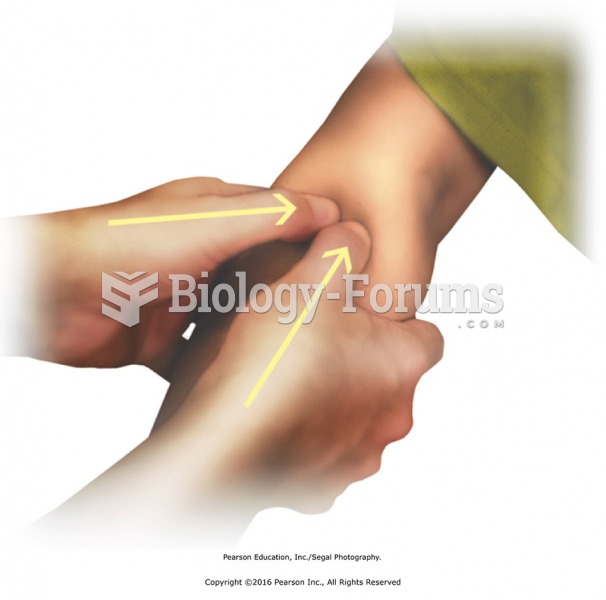 Apply thumb pressure to forearm muscles, particularly the extensors muscles. Apply circular friction ...
Apply thumb pressure to forearm muscles, particularly the extensors muscles. Apply circular friction ...