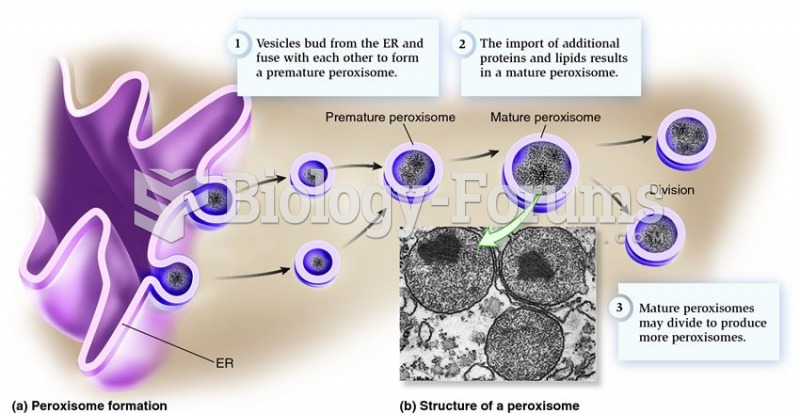
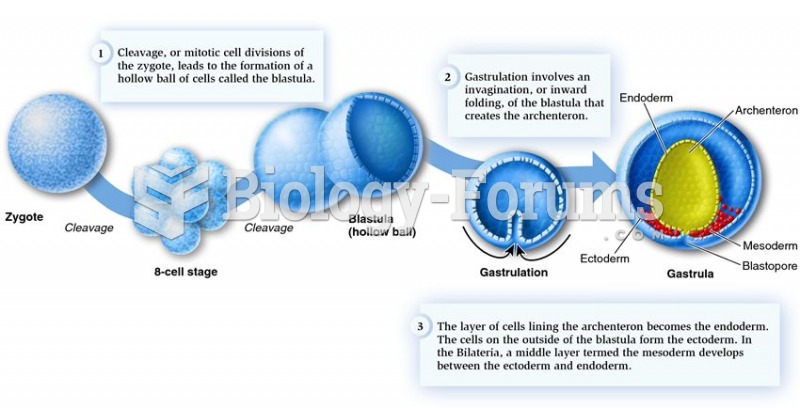
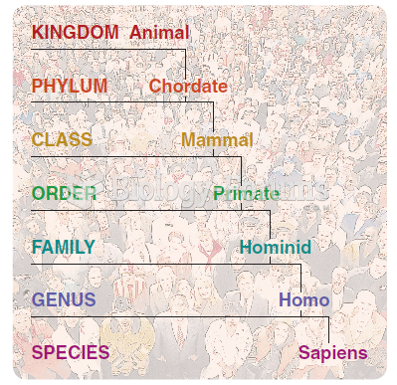
|
|
|
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
Only 12 hours after an egg cell is fertilized by a sperm cell, the egg cell starts to divide. As it continues to divide, it moves along the fallopian tube toward the uterus at about 1 inch per day.
Adults are resistant to the bacterium that causes Botulism. These bacteria thrive in honey – therefore, honey should never be given to infants since their immune systems are not yet resistant.
The Babylonians wrote numbers in a system that used 60 as the base value rather than the number 10. They did not have a symbol for "zero."
The use of salicylates dates back 2,500 years to Hippocrates’s recommendation of willow bark (from which a salicylate is derived) as an aid to the pains of childbirth. However, overdosage of salicylates can harm body fluids, electrolytes, the CNS, the GI tract, the ears, the lungs, the blood, the liver, and the kidneys and cause coma or death.