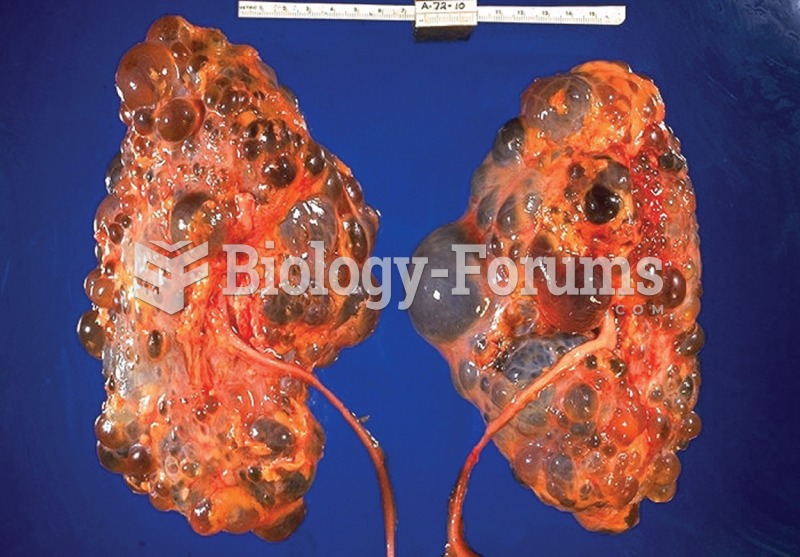
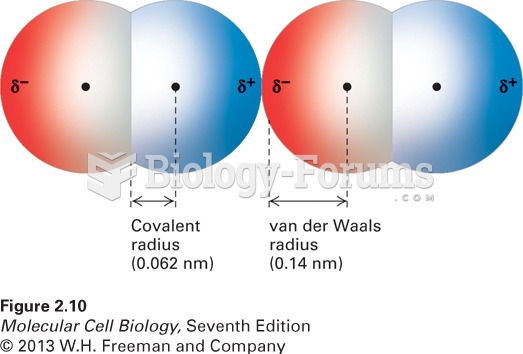
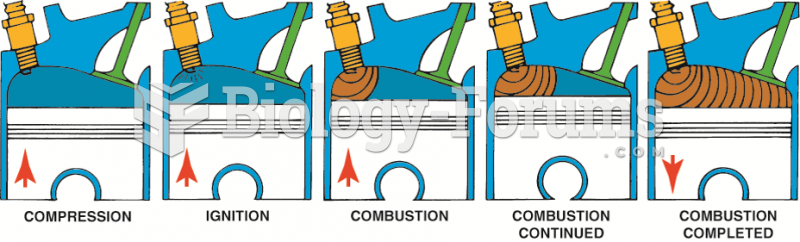
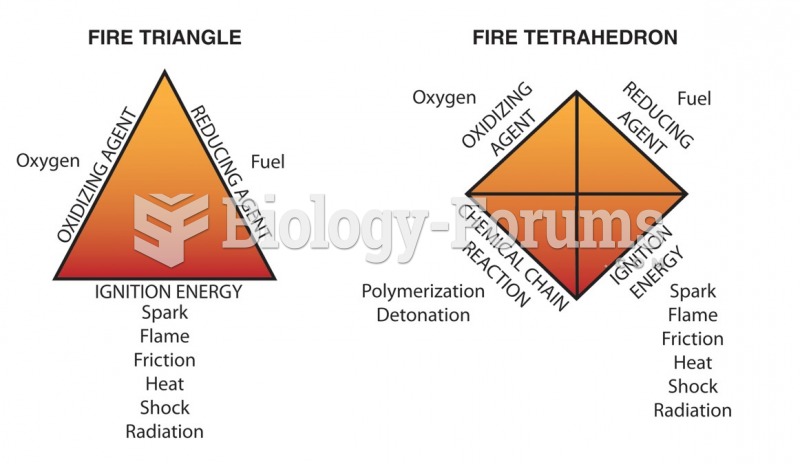
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
It is important to read food labels and choose foods with low cholesterol and saturated trans fat. You should limit saturated fat to no higher than 6% of daily calories.
Did you know?
If you could remove all of your skin, it would weigh up to 5 pounds.
Did you know?
Egg cells are about the size of a grain of sand. They are formed inside of a female's ovaries before she is even born.
Did you know?
Of the estimated 2 million heroin users in the United States, 600,000–800,000 are considered hardcore addicts. Heroin addiction is considered to be one of the hardest addictions to recover from.
Did you know?
Many of the drugs used by neuroscientists are derived from toxic plants and venomous animals (such as snakes, spiders, snails, and puffer fish).