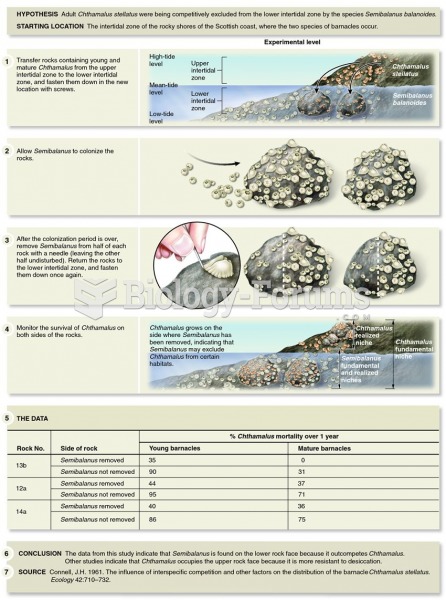
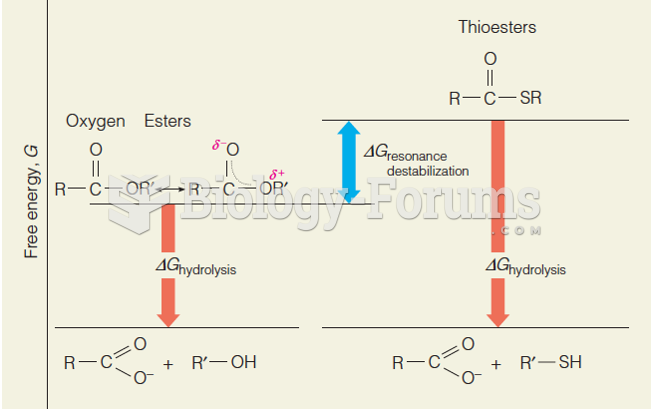
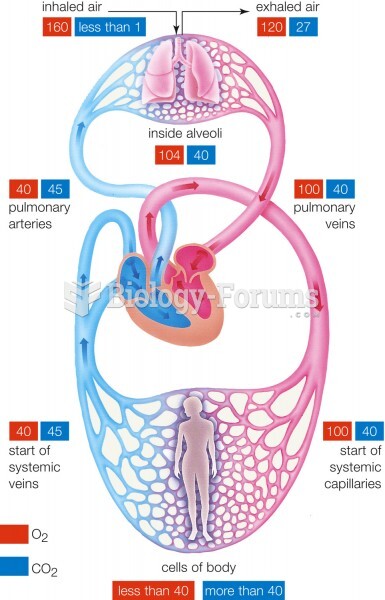
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Excessive alcohol use costs the country approximately $235 billion every year.
Did you know?
Opium has influenced much of the world's most popular literature. The following authors were all opium users, of varying degrees: Lewis Carroll, Charles, Dickens, Arthur Conan Doyle, and Oscar Wilde.
Did you know?
The Babylonians wrote numbers in a system that used 60 as the base value rather than the number 10. They did not have a symbol for "zero."
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
Serum cholesterol testing in adults is recommended every 1 to 5 years. People with diabetes and a family history of high cholesterol should be tested even more frequently.