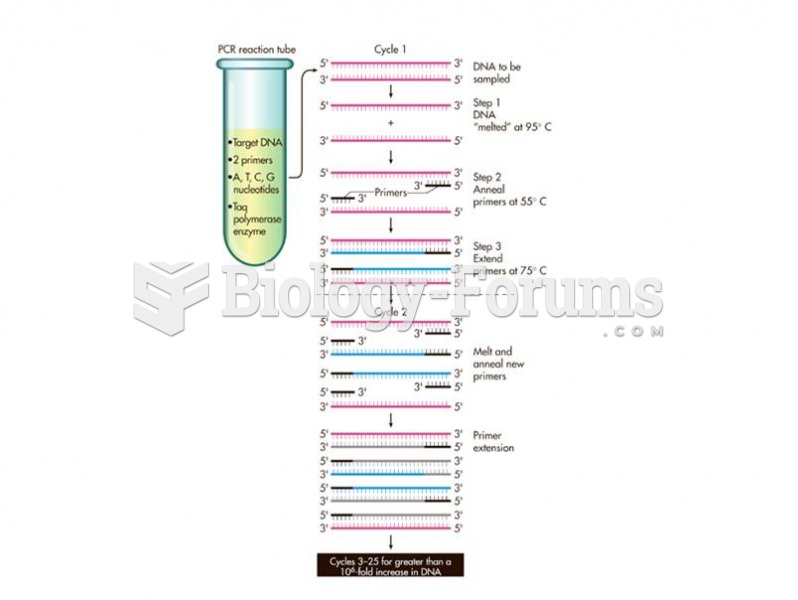
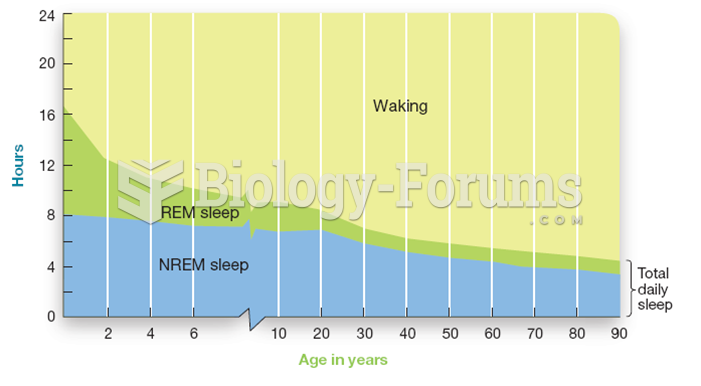
|
|
|
The people with the highest levels of LDL are Mexican American males and non-Hispanic black females.
Vampire bats have a natural anticoagulant in their saliva that permits continuous bleeding after they painlessly open a wound with their incisors. This capillary blood does not cause any significant blood loss to their victims.
Common abbreviations that cause medication errors include U (unit), mg (milligram), QD (every day), SC (subcutaneous), TIW (three times per week), D/C (discharge or discontinue), HS (at bedtime or "hours of sleep"), cc (cubic centimeters), and AU (each ear).
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.
When blood is exposed to air, it clots. Heparin allows the blood to come in direct contact with air without clotting.