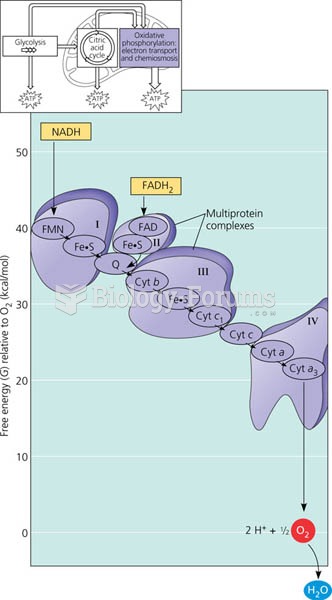
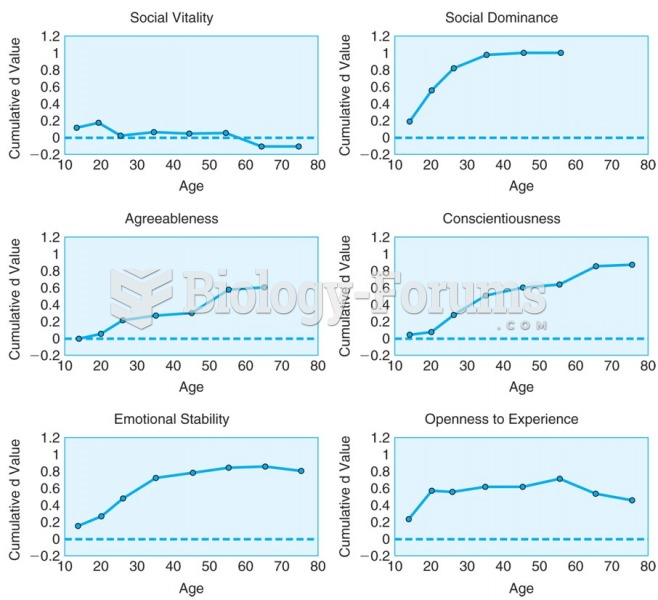
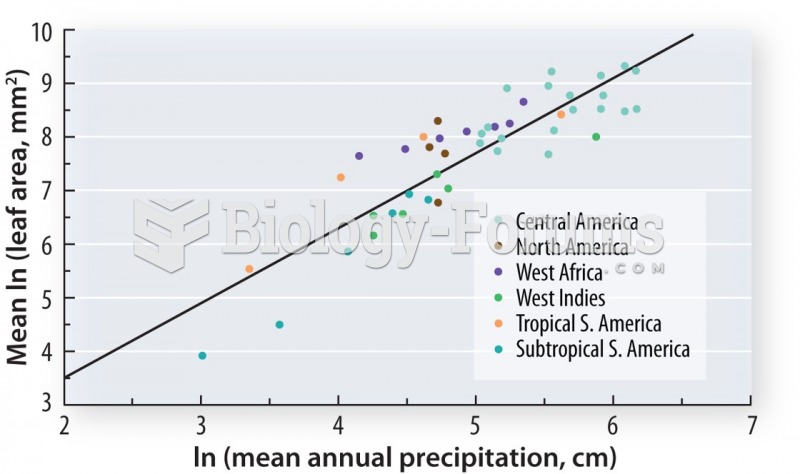
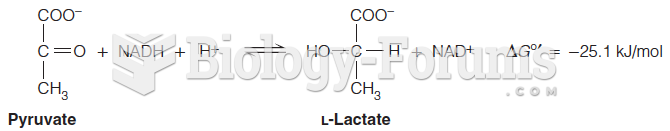|
|
|
About 60% of newborn infants in the United States are jaundiced; that is, they look yellow. Kernicterus is a form of brain damage caused by excessive jaundice. When babies begin to be affected by excessive jaundice and begin to have brain damage, they become excessively lethargic.
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.
The average person is easily confused by the terms pharmaceutics and pharmacology, thinking they are one and the same. Whereas pharmaceutics is the science of preparing and dispensing drugs (otherwise known as the science of pharmacy), pharmacology is the study of medications.
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.
The FDA recognizes 118 routes of administration.