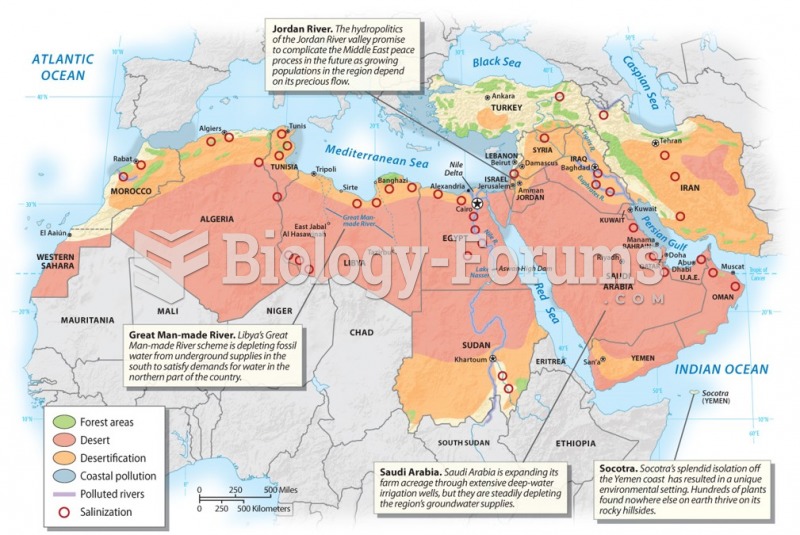
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Medications that are definitely not safe to take when breastfeeding include radioactive drugs, antimetabolites, some cancer (chemotherapy) agents, bromocriptine, ergotamine, methotrexate, and cyclosporine.
Did you know?
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
Did you know?
Adults are resistant to the bacterium that causes Botulism. These bacteria thrive in honey – therefore, honey should never be given to infants since their immune systems are not yet resistant.
Did you know?
There are more bacteria in your mouth than there are people in the world.
Did you know?
More than 150,000 Americans killed by cardiovascular disease are younger than the age of 65 years.