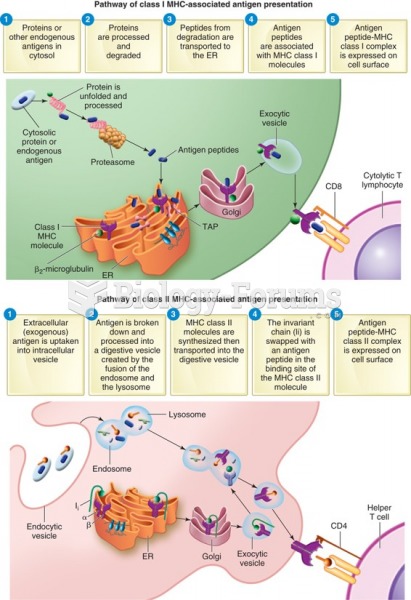
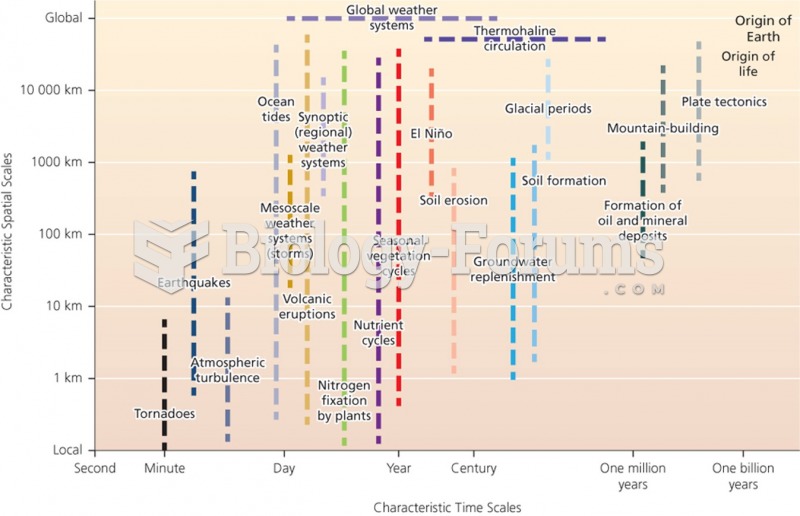
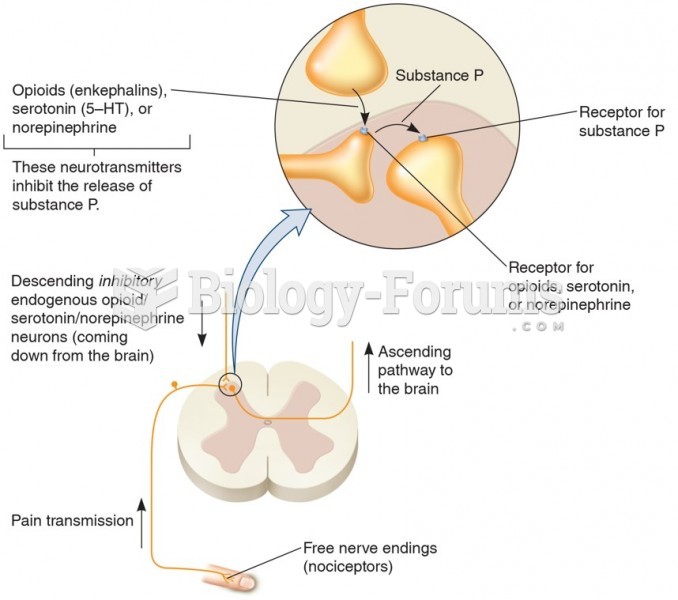
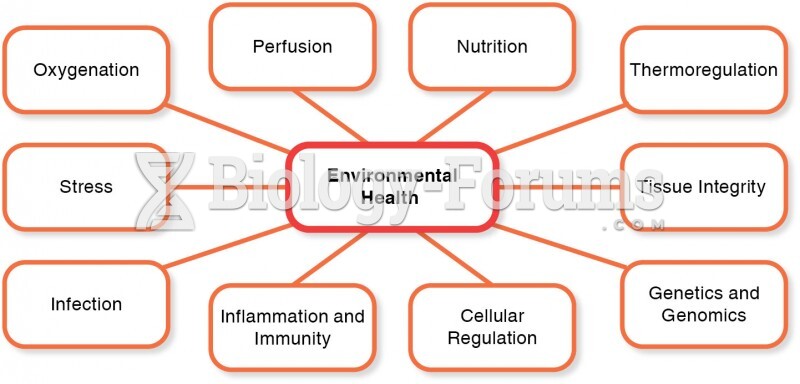
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
About 600,000 particles of skin are shed every hour by each human. If you live to age 70 years, you have shed 105 pounds of dead skin.
Did you know?
More than 30% of American adults, and about 12% of children utilize health care approaches that were developed outside of conventional medicine.
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.
Did you know?
Medication errors are three times higher among children and infants than with adults.
Did you know?
There are more sensory neurons in the tongue than in any other part of the body.