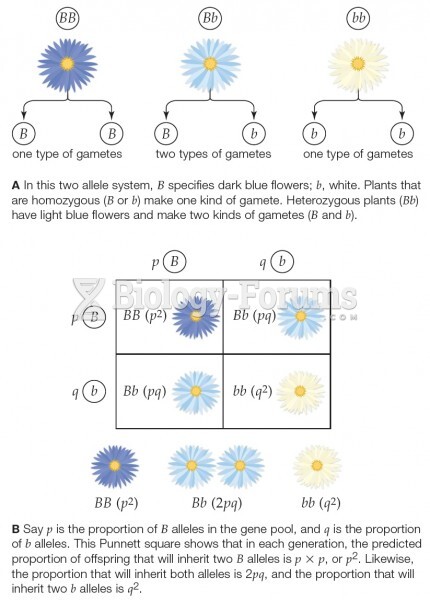
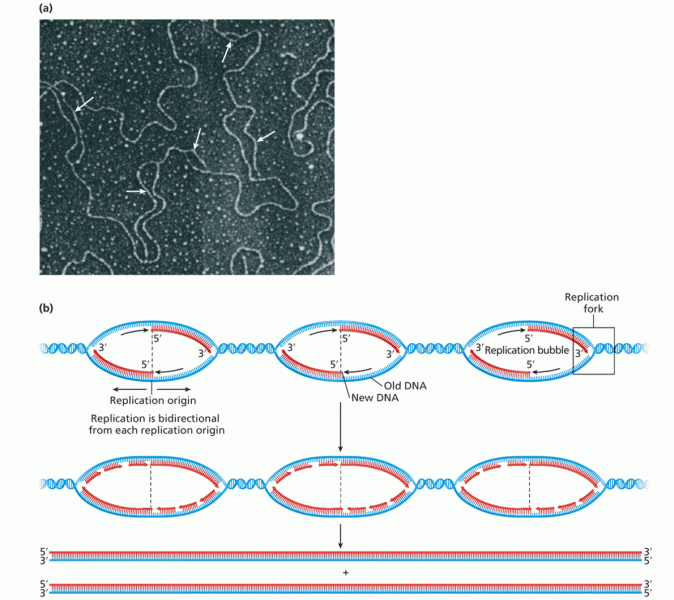
|
|
|
Did you know?
Increased intake of vitamin D has been shown to reduce fractures up to 25% in older people.
Did you know?
Many supplement containers do not even contain what their labels say. There are many documented reports of products containing much less, or more, that what is listed on their labels. They may also contain undisclosed prescription drugs and even contaminants.
Did you know?
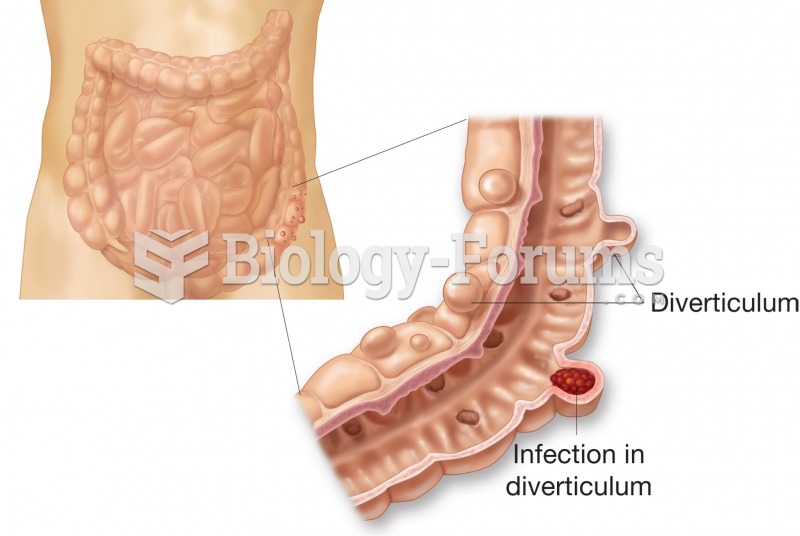
Many medications that are used to treat infertility are injected subcutaneously. This is easy to do using the anterior abdomen as the site of injection but avoiding the area directly around the belly button.
Did you know?
The FDA recognizes 118 routes of administration.
Did you know?
There are more sensory neurons in the tongue than in any other part of the body.