|
|
|
There are over 65,000 known species of protozoa. About 10,000 species are parasitic.
The Food and Drug Administration has approved Risperdal, an adult antipsychotic drug, for the symptomatic treatment of irritability in children and adolescents with autism. The approval is the first for the use of a drug to treat behaviors associated with autism in children. These behaviors are included under the general heading of irritability and include aggression, deliberate self-injury, and temper tantrums.
There are actually 60 minerals, 16 vitamins, 12 essential amino acids, and three essential fatty acids that your body needs every day.
To prove that stomach ulcers were caused by bacteria and not by stress, a researcher consumed an entire laboratory beaker full of bacterial culture. After this, he did indeed develop stomach ulcers, and won the Nobel Prize for his discovery.
Most fungi that pathogenically affect humans live in soil. If a person is not healthy, has an open wound, or is immunocompromised, a fungal infection can be very aggressive.
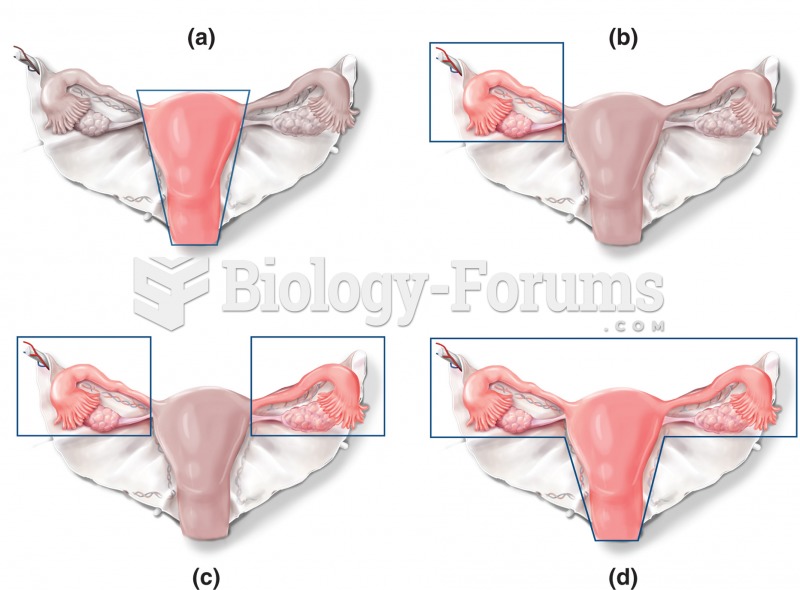 Alternative forms of surgeries involving the uterus, ovaries, and fallopian tubes. The solid lines i
Alternative forms of surgeries involving the uterus, ovaries, and fallopian tubes. The solid lines i
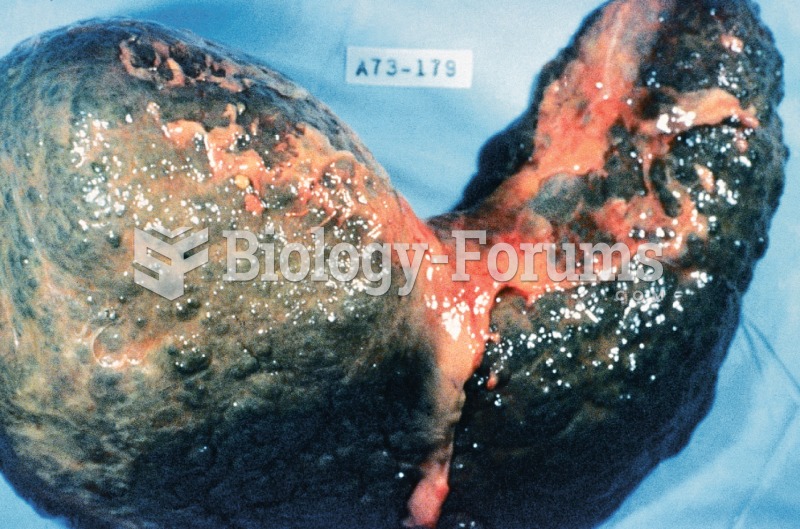 Cirrhosis. Cirrhosis is characterized by a chronic deterioration of the liver, replacing healthy cel
Cirrhosis. Cirrhosis is characterized by a chronic deterioration of the liver, replacing healthy cel