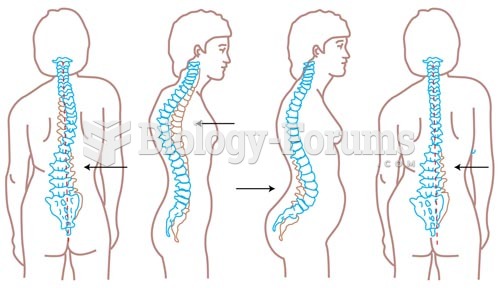
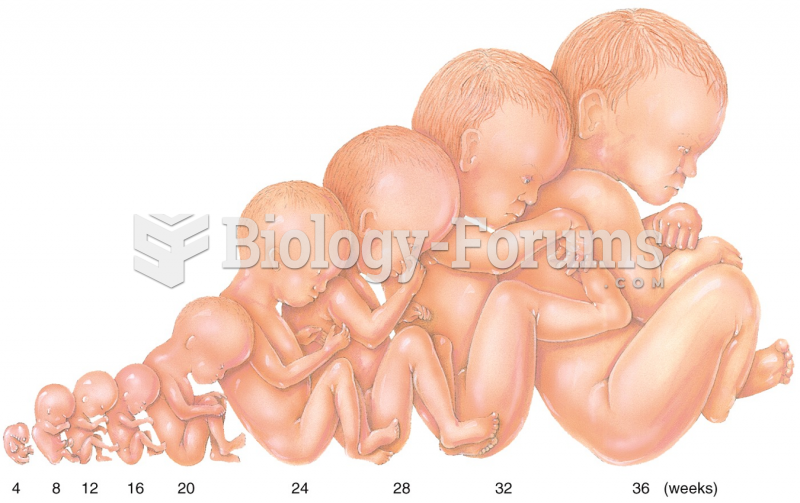
|
|
|
By definition, when a medication is administered intravenously, its bioavailability is 100%.
When blood is exposed to air, it clots. Heparin allows the blood to come in direct contact with air without clotting.
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.
Illness; diuretics; laxative abuse; hot weather; exercise; sweating; caffeine; alcoholic beverages; starvation diets; inadequate carbohydrate consumption; and diets high in protein, salt, or fiber can cause people to become dehydrated.
Looking at the sun may not only cause headache and distort your vision temporarily, but it can also cause permanent eye damage. Any exposure to sunlight adds to the cumulative effects of ultraviolet (UV) radiation on your eyes. UV exposure has been linked to eye disorders such as macular degeneration, solar retinitis, and corneal dystrophies.