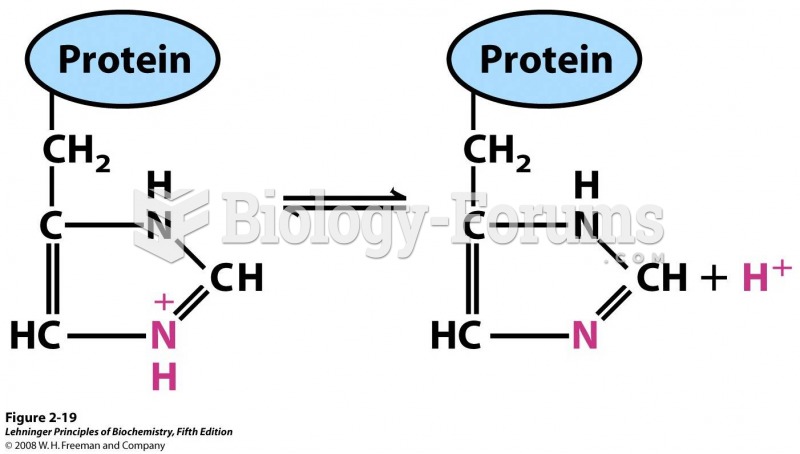
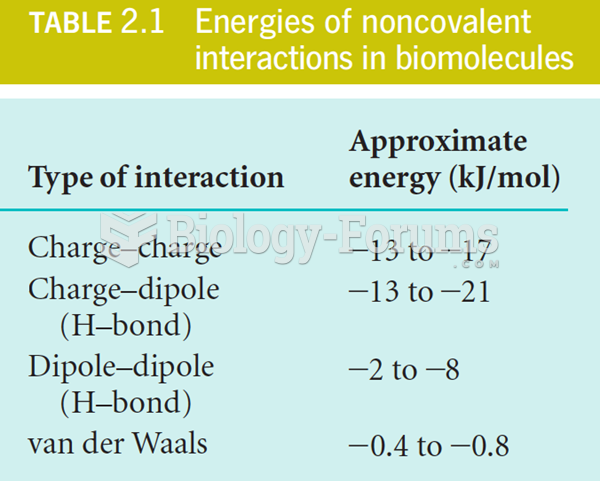
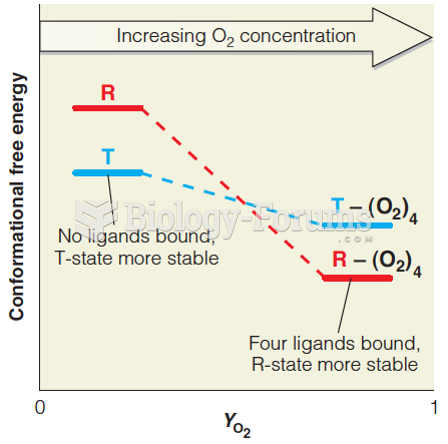
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The average adult has about 21 square feet of skin.
Did you know?
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.
Did you know?
As of mid-2016, 18.2 million people were receiving advanced retroviral therapy (ART) worldwide. This represents between 43–50% of the 34–39.8 million people living with HIV.
Did you know?
About one in five American adults and teenagers have had a genital herpes infection—and most of them don't know it. People with genital herpes have at least twice the risk of becoming infected with HIV if exposed to it than those people who do not have genital herpes.
Did you know?
By definition, when a medication is administered intravenously, its bioavailability is 100%.