|
|
|
Astigmatism is the most common vision problem. It may accompany nearsightedness or farsightedness. It is usually caused by an irregularly shaped cornea, but sometimes it is the result of an irregularly shaped lens. Either type can be corrected by eyeglasses, contact lenses, or refractive surgery.
Though Candida and Aspergillus species are the most common fungal pathogens causing invasive fungal disease in the immunocompromised, infections due to previously uncommon hyaline and dematiaceous filamentous fungi are occurring more often today. Rare fungal infections, once accurately diagnosed, may require surgical debridement, immunotherapy, and newer antifungals used singly or in combination with older antifungals, on a case-by-case basis.
The average human gut is home to perhaps 500 to 1,000 different species of bacteria.
There are over 65,000 known species of protozoa. About 10,000 species are parasitic.
The heart is located in the center of the chest, with part of it tipped slightly so that it taps against the left side of the chest.
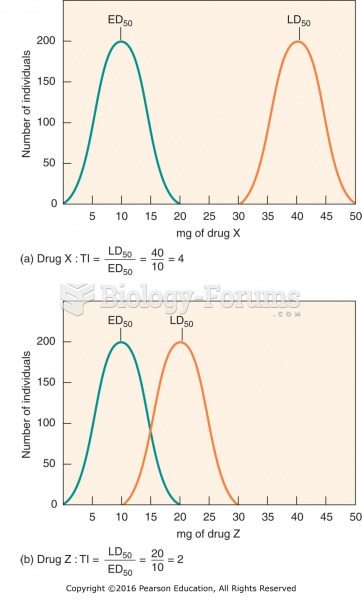 Therapeutic index: (a) Drug X has a therapeutic index of 4. (b) Drug Z has a therapeutic index of 2.
Therapeutic index: (a) Drug X has a therapeutic index of 4. (b) Drug Z has a therapeutic index of 2.
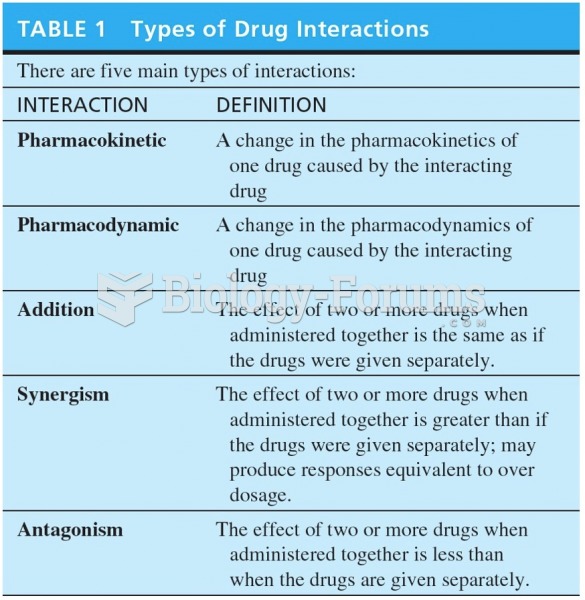
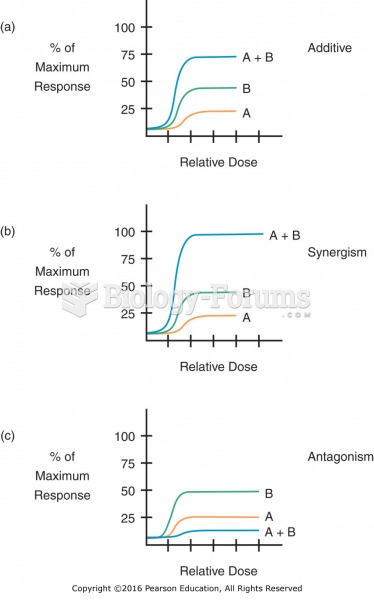 Additive, synergistic, and antagonistic drug interactions: (a) additive response; (b) synergistic ...
Additive, synergistic, and antagonistic drug interactions: (a) additive response; (b) synergistic ...