Answer to Question 1
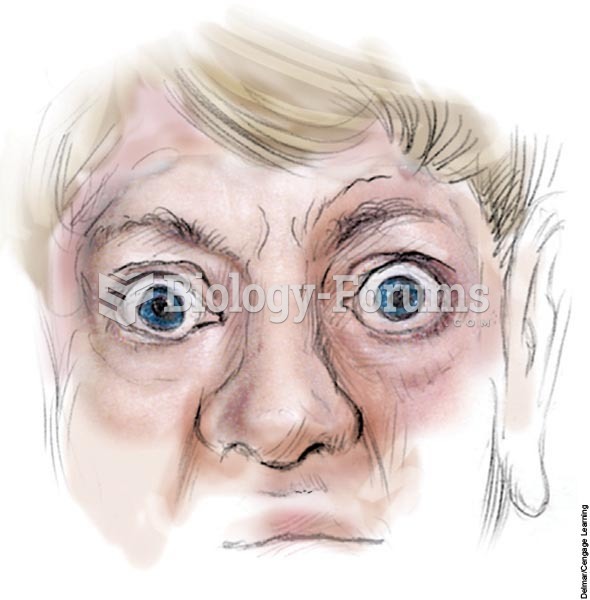
The main symptoms of Parkinson's disease (also known as Parkinson disease) are rigidity, muscle tremors, slow movements, and difficulty initiating physical and mental activity. It becomes more common as people age, striking 1 percent to 2 percent of people over age 65 . Early symptoms usually include loss of olfaction and psychological depression. Many but not all Parkinson's patients have cognitive deficits, which may include problems with attention, language, or memory. The immediate cause of Parkinson's disease is the gradual loss of neurons in the substantia nigra and therefore a loss of dopamine-releasing axons to the striatum (part of the basal ganglia). With the loss of this input, the striatum decreases its inhibition of the globus pallidus, which therefore increases its inhibitory input to the thalamus. The result is less vigorous voluntary movements. People with Parkinson's disease are still capable of movement, and sometimes they move normally in response to signals or instructions, such as when following a parade. However, their spontaneous movements are slow and weak.
What causes the damage to the substantia nigra? An early study reported that having a monozygotic twin with early-onset Parkinson's disease greatly increased your probability of getting it, but having a monozygotic twin with late-onset disease had no effect. That result implied that genes make little or no contribution to late-onset Parkinson's disease. Later studies have found less extreme results, indicating that genes do influence the late-onset disease, though less strongly than they impact early-onset disease. So far, researchers have identified more than 20 genes that apparently increase the risk of Parkinson's disease, although the results vary from one study to another, and one population to another.
The results agree, however, that none of these genes by itself produces a high risk. An accidental discovery implicated exposure to toxins as another factor in Parkinson's disease. In northern California in 1982, several young adults developed symptoms of Parkinson's disease after using a drug similar to heroin. Before the investigators could alert the community to the danger, many other users had developed symptoms ranging from mild to fatal. The substance responsible for the symptoms was MPTP, a chemical that the body converts to MPP+, which accumulates in, and then destroys, neurons that release dopamine, partly by impairing the transport of mitochondria from the cell body to the synapse. Postsynaptic neurons react to the loss of input by increasing their number of dopamine receptors.
People are sometimes exposed to hazardous environmental chemicals that damage cells of the substantia nigra. Many studies have shown increased risk of Parkinson's disease among people with much exposure to insecticides, herbicides, and fungicides, including paraquat, rotenone, maneb, and ziram. The disease is more common in farmers and other rural dwellers than in city dwellers, presumably because of increased exposure to these chemicals. Exposure to these chemicals increases the risk especially among people with any of the genes that predispose to Parkinson's. If someone also had a traumatic head injury, the risk goes up even more. In short, most cases result from several influences combined, not just one.
What else might influence the risk of Parkinson's disease? Researchers compared the lifestyles of people who did and didn't develop the disease. One factor that stands out consistently is cigarette smoking and coffee drinking: People who smoke cigarettes or drink coffee have less chance of developing Parkinson's disease.
Because Parkinson's disease results from a dopamine deficiency, a logical goal is to restore the missing dopamine. A dopamine pill would be ineffective because dopamine does not cross the bloodbrain barrier. Physicians in the 1950s and 1960s reasoned that L-dopa, a precursor to dopamine that does cross the barrier, might be a good treatment. In contrast to all the medicines that were discovered by trial and error, this was the first drug in psychiatry or neurology, and one of the first in all of medicine, to emerge from a plausible theory. Taken as a daily pill, L-dopa reaches the brain, where neurons convert it to dopamine. L-dopa is still the most common treatment for Parkinson's disease. However, L-dopa treatment is disappointing in several ways. It increases dopamine release in all axons, including those that had deteriorated and those that were still functioning normally. It produces spurts of high release alternating with lower release. Even if it adequately replaces lost dopamine, it does not replace other transmitters that are also depleted. It does not slow the continuing loss of neurons. And it produces unpleasant side effects such as nausea, restlessness, sleep problems, low blood pressure, repetitive movements, and sometimes hallucinations and delusions.
A potentially exciting strategy has been in the experimental stage since the 1980s. In a pioneering study, M. J. Perlow and colleagues (1979) injected the chemical 6-OHDA (6-hydroxydopamine, a chemical modification of dopamine) into rats to damage the substantia nigra of one hemisphere, producing Parkinson's-type symptoms on the opposite side of the body. After the movement abnormalities stabilized, the experimenters transplanted substantia nigra tissue from rat fetuses into the damaged brains. Most recipients recovered much of their normal movement within four weeks. Control animals that suffered the same brain damage without receiving grafts showed little or no recovery. This is only a partial brain transplant, but still, the Frankensteinian implications are striking.
If such surgery works for rats, might it also for humans? Ordinarily, scientists test any experimental procedure extensively with laboratory animals before trying it on humans, but with Parkinson's disease, the temptation was too great. People in the late stages have little to lose and are willing to try almost anything. The obvious problem is where to get the donor tissue. Several early studies used tissue from the patient's own adrenal gland. Although that tissue is not composed of neurons, it produces and releases dopamine. Unfortunately, the adrenal gland transplants seldom produced much benefit.
Another possibility is to transplant brain tissue from aborted fetuses. Fetal neurons transplanted into the brains of patients with Parkinson's sometimes survive for years and make synapses with the patient's own cells. However, the operation is expensive and difficult, requiring brain tissue from four to eight aborted fetuses, and the benefits to the patient have been small at best.
A related approach is to take stem cellsimmature cells that are capable of differentiating into other cell typesguide their development so that they produce large quantities of L-dopa, and then transplant them into the brain. The idea sounds promising, but researchers will need to overcome several difficulties before this might become an effective treatment.
Answer to Question 2
b