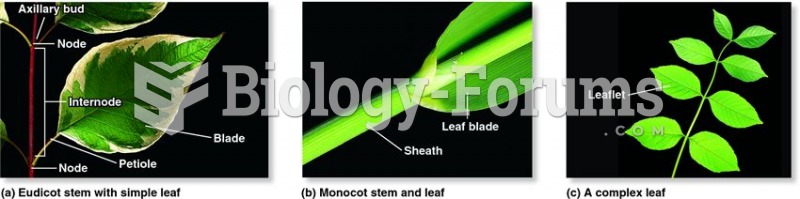
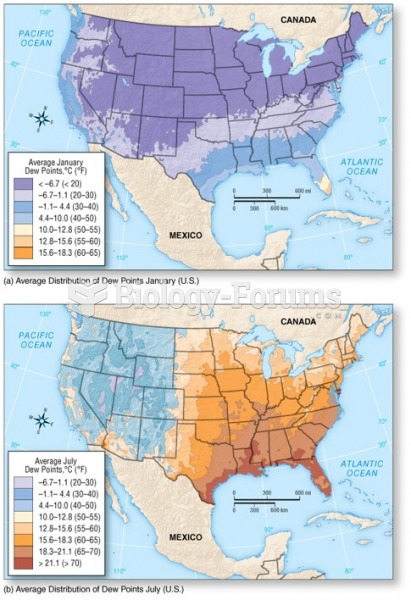
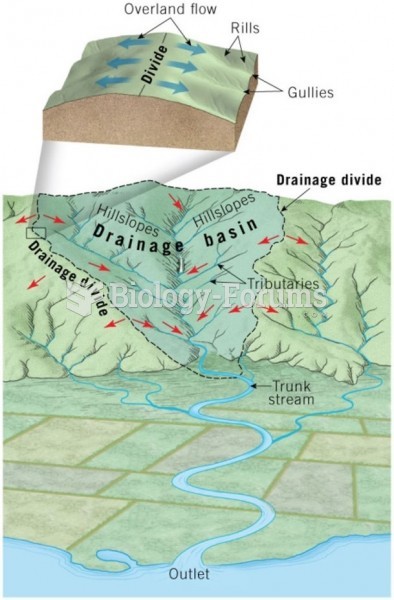
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The first oral chemotherapy drug for colon cancer was approved by FDA in 2001.
Did you know?
Asthma is the most common chronic childhood disease in the world. Most children who develop asthma have symptoms before they are 5 years old.
Did you know?
There are 20 feet of blood vessels in each square inch of human skin.
Did you know?
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
Did you know?
When intravenous medications are involved in adverse drug events, their harmful effects may occur more rapidly, and be more severe than errors with oral medications. This is due to the direct administration into the bloodstream.