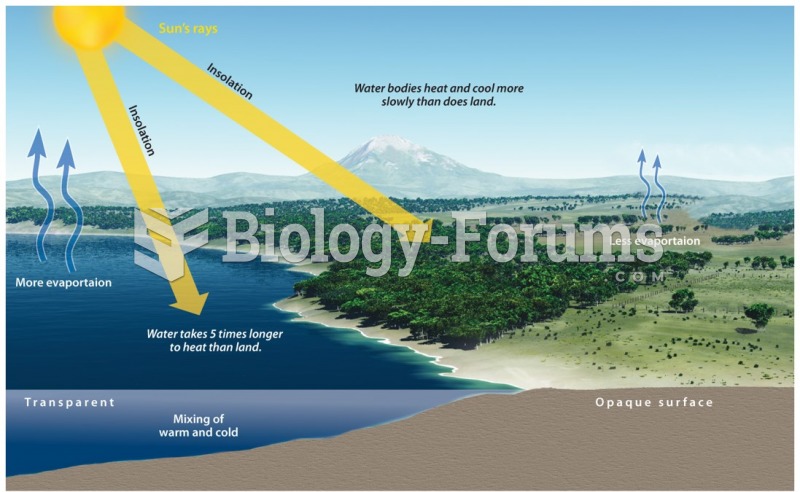
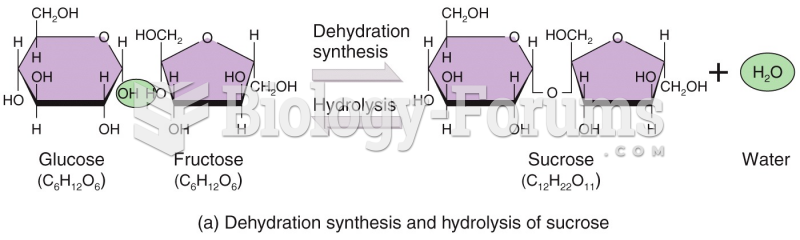
|
|
|
There are more nerve cells in one human brain than there are stars in the Milky Way.
Human kidneys will clean about 1 million gallons of blood in an average lifetime.
In 2012, nearly 24 milliion Americans, aged 12 and older, had abused an illicit drug, according to the National Institute on Drug Abuse (NIDA).
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
People who have myopia, or nearsightedness, are not able to see objects at a distance but only up close. It occurs when the cornea is either curved too steeply, the eye is too long, or both. This condition is progressive and worsens with time. More than 100 million people in the United States are nearsighted, but only 20% of those are born with the condition. Diet, eye exercise, drug therapy, and corrective lenses can all help manage nearsightedness.