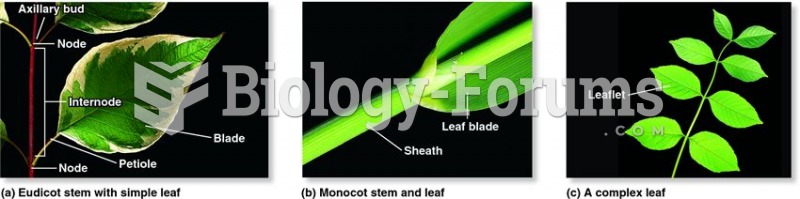
|
|
|
Women are 50% to 75% more likely than men to experience an adverse drug reaction.
A serious new warning has been established for pregnant women against taking ACE inhibitors during pregnancy. In the study, the risk of major birth defects in children whose mothers took ACE inhibitors during the first trimester was nearly three times higher than in children whose mothers didn't take ACE inhibitors. Physicians can prescribe alternative medications for pregnant women who have symptoms of high blood pressure.
Of the estimated 2 million heroin users in the United States, 600,000–800,000 are considered hardcore addicts. Heroin addiction is considered to be one of the hardest addictions to recover from.
In 2012, nearly 24 milliion Americans, aged 12 and older, had abused an illicit drug, according to the National Institute on Drug Abuse (NIDA).
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.