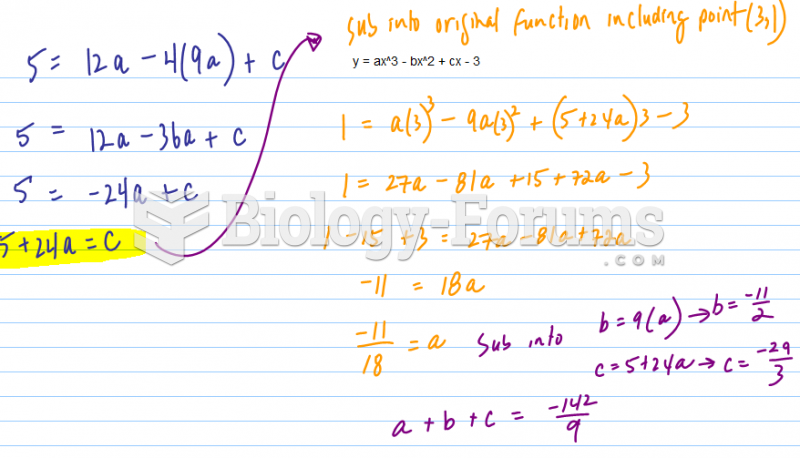
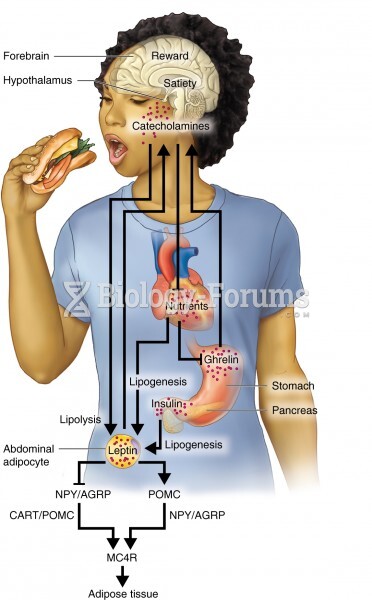
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Adolescents often feel clumsy during puberty because during this time of development, their hands and feet grow faster than their arms and legs do. The body is therefore out of proportion. One out of five adolescents actually experiences growing pains during this period.
Did you know?
Oliver Wendell Holmes is credited with introducing the words "anesthesia" and "anesthetic" into the English language in 1846.
Did you know?
The people with the highest levels of LDL are Mexican American males and non-Hispanic black females.
Did you know?
The horizontal fraction bar was introduced by the Arabs.
Did you know?
Green tea is able to stop the scent of garlic or onion from causing bad breath.