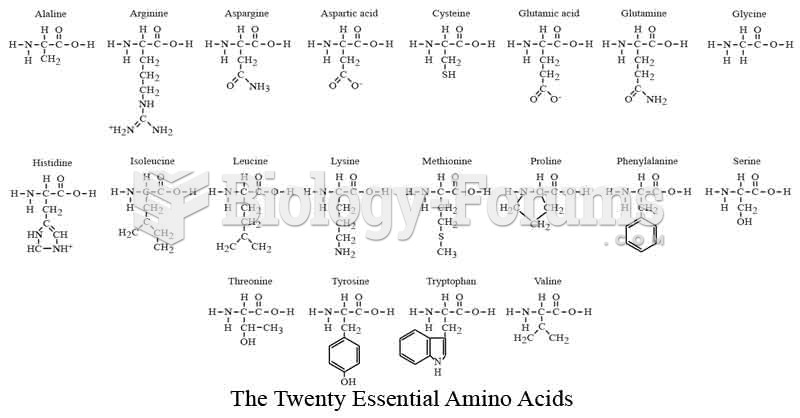
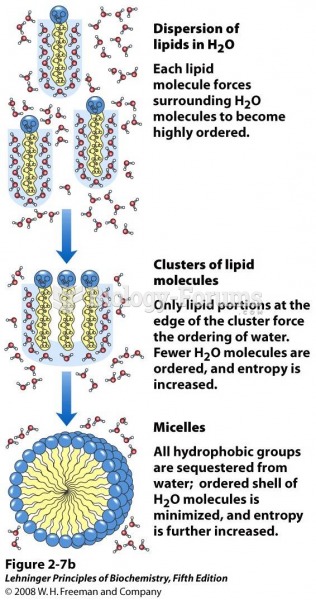
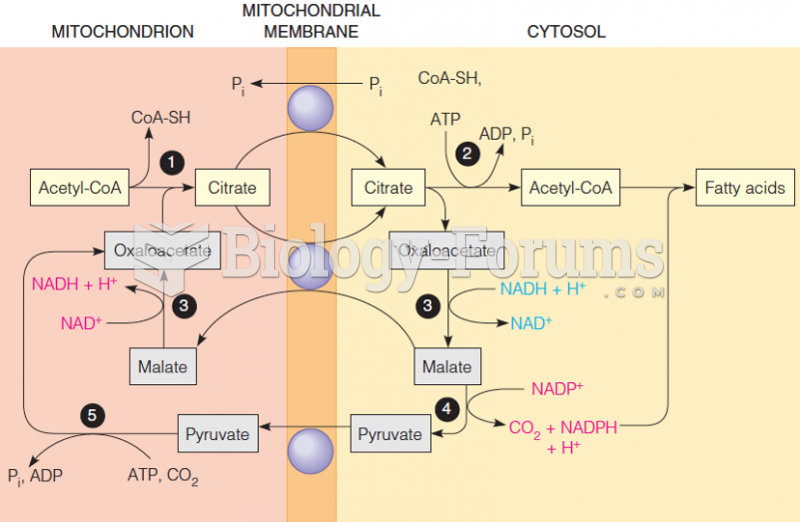
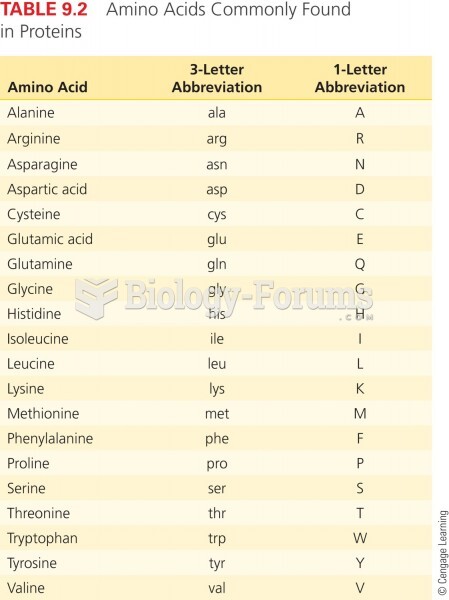
|
|
|
More than 4.4billion prescriptions were dispensed within the United States in 2016.
Of the estimated 2 million heroin users in the United States, 600,000–800,000 are considered hardcore addicts. Heroin addiction is considered to be one of the hardest addictions to recover from.
When blood is deoxygenated and flowing back to the heart through the veins, it is dark reddish-blue in color. Blood in the arteries that is oxygenated and flowing out to the body is bright red. Whereas arterial blood comes out in spurts, venous blood flows.
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
Cytomegalovirus affects nearly the same amount of newborns every year as Down syndrome.