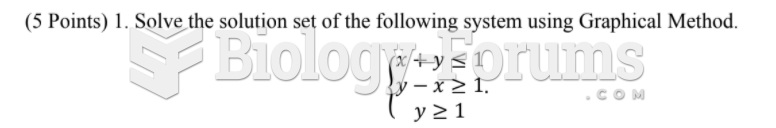This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The ratio of hydrogen atoms to oxygen in water (H2O) is 2:1.
Did you know?
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.
Did you know?
Calcitonin is a naturally occurring hormone. In women who are at least 5 years beyond menopause, it slows bone loss and increases spinal bone density.
Did you know?
Critical care patients are twice as likely to receive the wrong medication. Of these errors, 20% are life-threatening, and 42% require additional life-sustaining treatments.
Did you know?
Street names for barbiturates include reds, red devils, yellow jackets, blue heavens, Christmas trees, and rainbows. They are commonly referred to as downers.