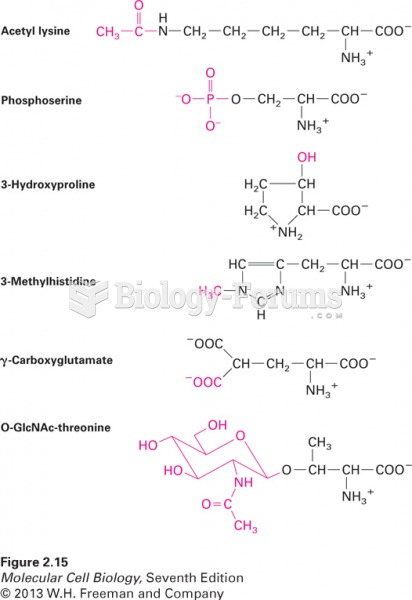
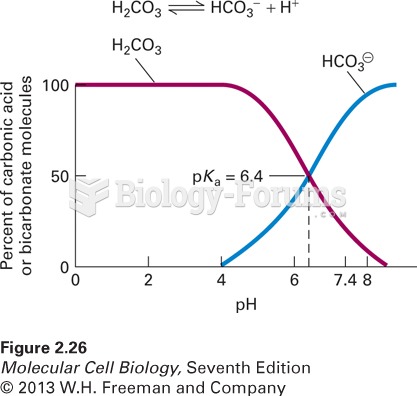
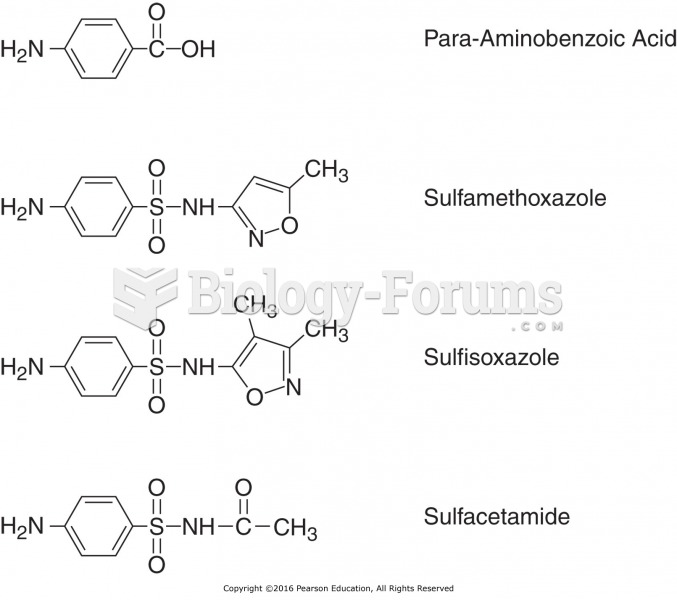
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Fungal nail infections account for up to 30% of all skin infections. They affect 5% of the general population—mostly people over the age of 70.
Did you know?
In 2010, opiate painkllers, such as morphine, OxyContin®, and Vicodin®, were tied to almost 60% of drug overdose deaths.
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.
Did you know?
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.