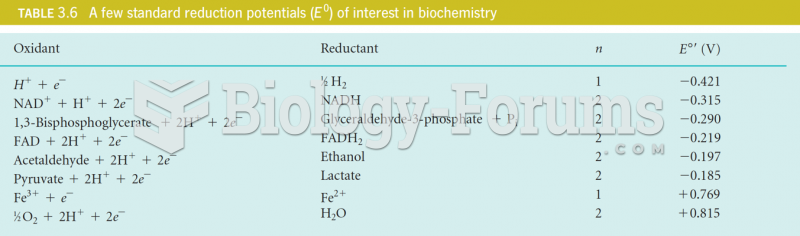
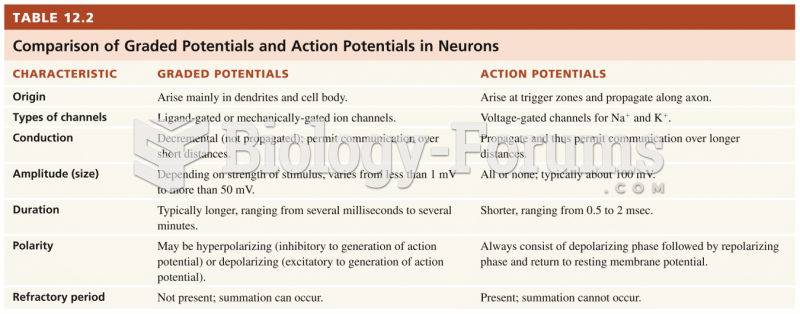
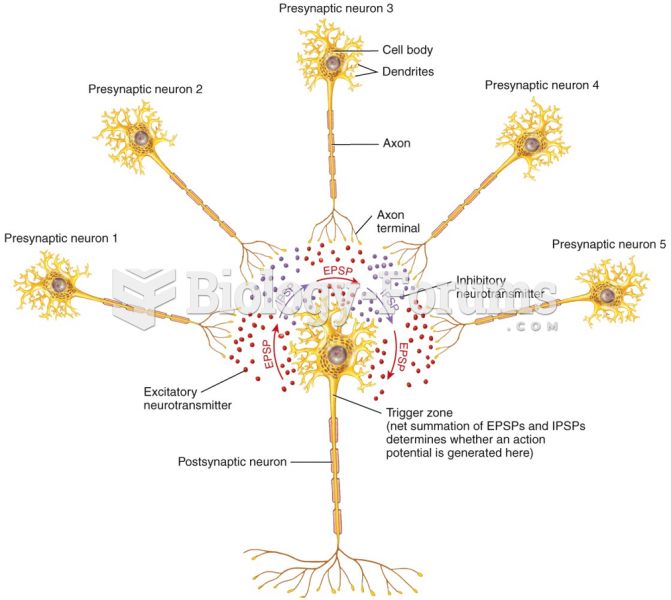
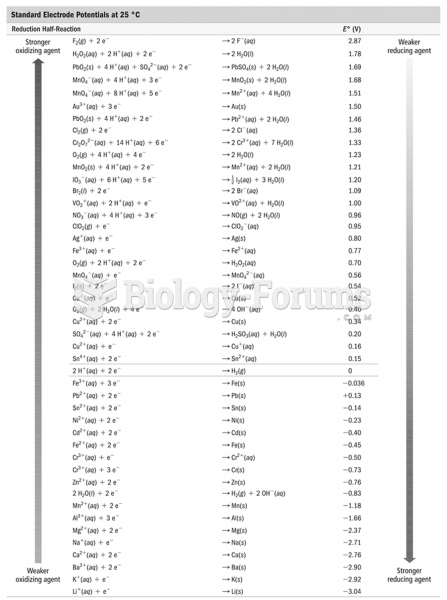
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Eat fiber! A diet high in fiber can help lower cholesterol levels by as much as 10%.
Did you know?
Green tea is able to stop the scent of garlic or onion from causing bad breath.
Did you know?
Malaria was not eliminated in the United States until 1951. The term eliminated means that no new cases arise in a country for 3 years.
Did you know?
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
Did you know?
When blood is exposed to air, it clots. Heparin allows the blood to come in direct contact with air without clotting.