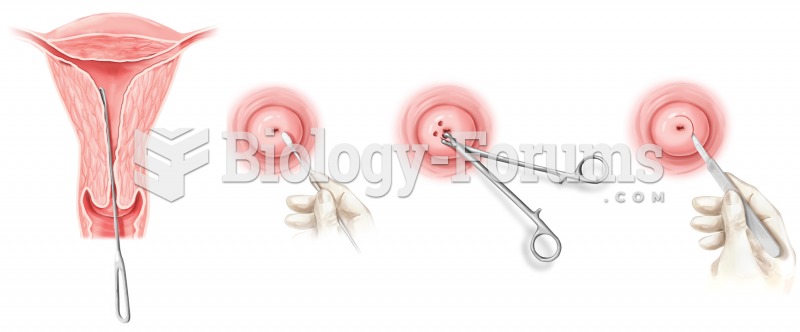
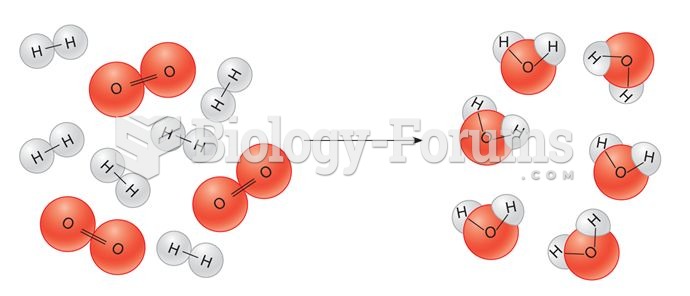
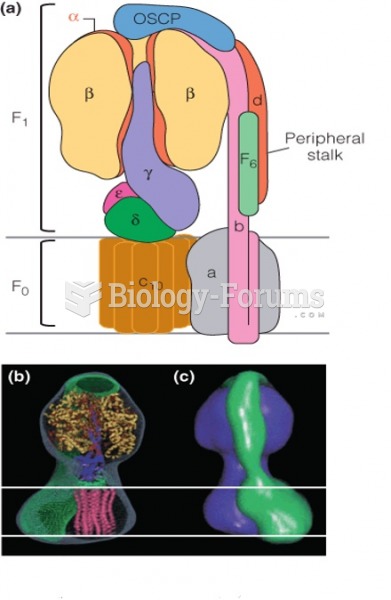
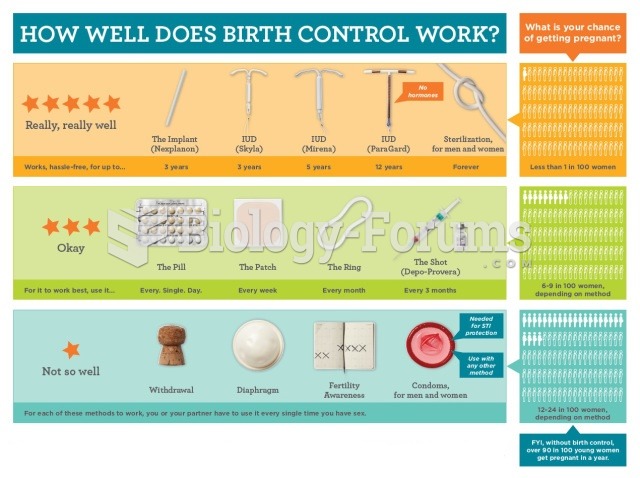
|
|
|
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
Vaccines prevent between 2.5 and 4 million deaths every year.
Drugs are in development that may cure asthma and hay fever once and for all. They target leukotrienes, which are known to cause tightening of the air passages in the lungs and increase mucus productions in nasal passages.
A recent study has found that following a diet rich in berries may slow down the aging process of the brain. This diet apparently helps to keep dopamine levels much higher than are seen in normal individuals who do not eat berries as a regular part of their diet as they enter their later years.
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.