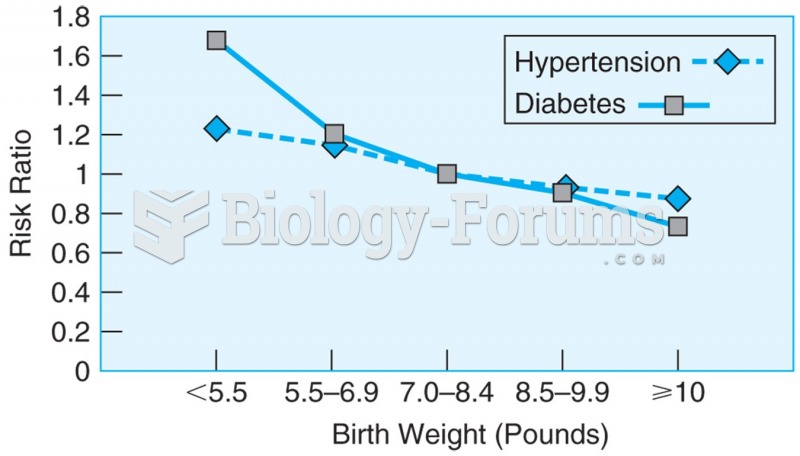
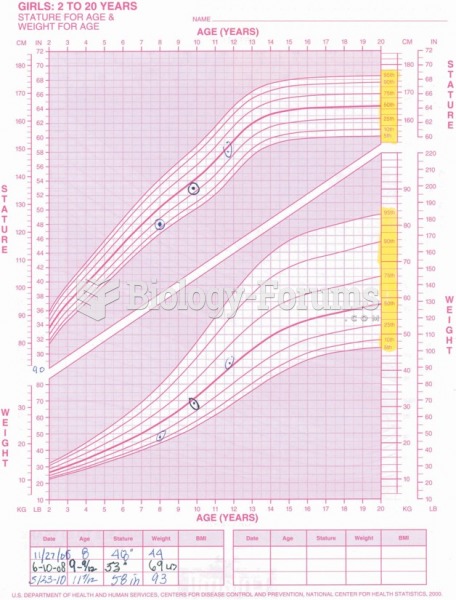
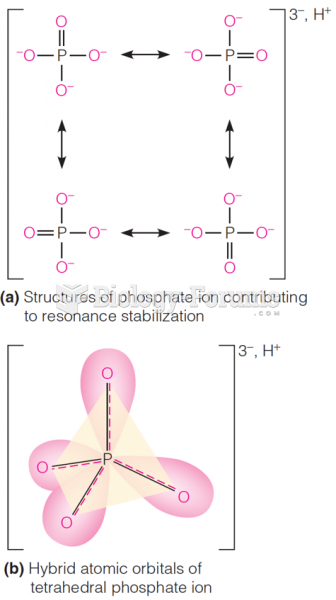
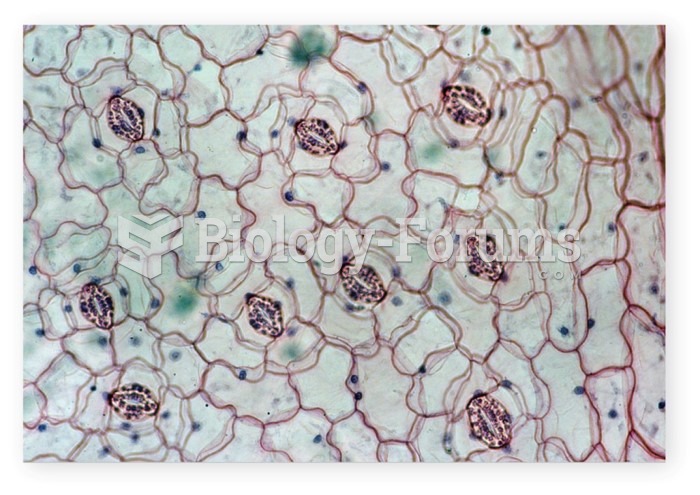
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are more bacteria in your mouth than there are people in the world.
Did you know?
Everyone has one nostril that is larger than the other.
Did you know?
Blood is approximately twice as thick as water because of the cells and other components found in it.
Did you know?
Egg cells are about the size of a grain of sand. They are formed inside of a female's ovaries before she is even born.
Did you know?
In the United States, congenital cytomegalovirus causes one child to become disabled almost every hour. CMV is the leading preventable viral cause of development disability in newborns. These disabilities include hearing or vision loss, and cerebral palsy.