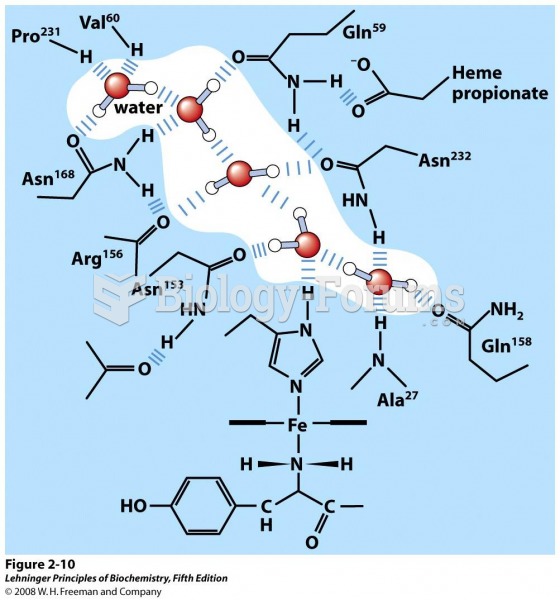
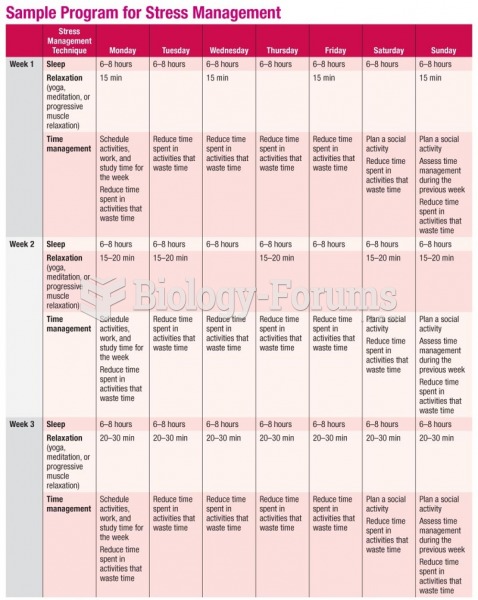
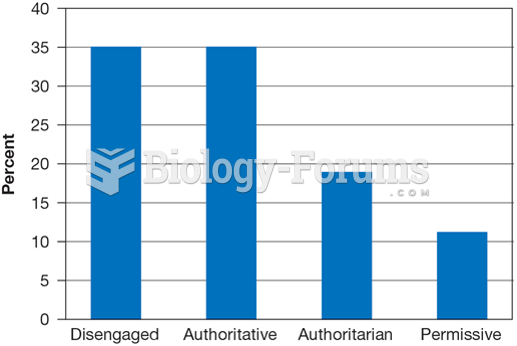
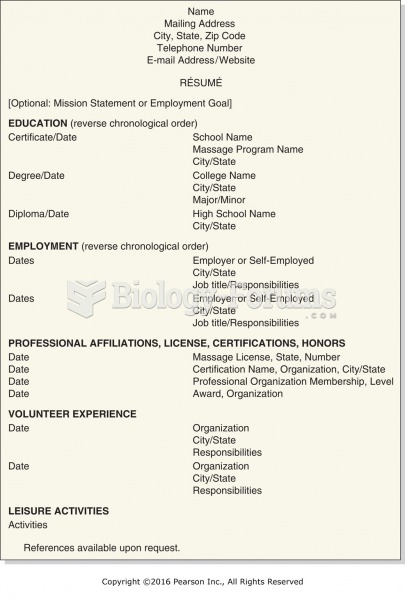
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Your heart beats over 36 million times a year.
Did you know?
Multiple sclerosis is a condition wherein the body's nervous system is weakened by an autoimmune reaction that attacks the myelin sheaths of neurons.
Did you know?
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
Did you know?
Women are two-thirds more likely than men to develop irritable bowel syndrome. This may be attributable to hormonal changes related to their menstrual cycles.
Did you know?
For high blood pressure (hypertension), a new class of drug, called a vasopeptidase blocker (inhibitor), has been developed. It decreases blood pressure by simultaneously dilating the peripheral arteries and increasing the body's loss of salt.