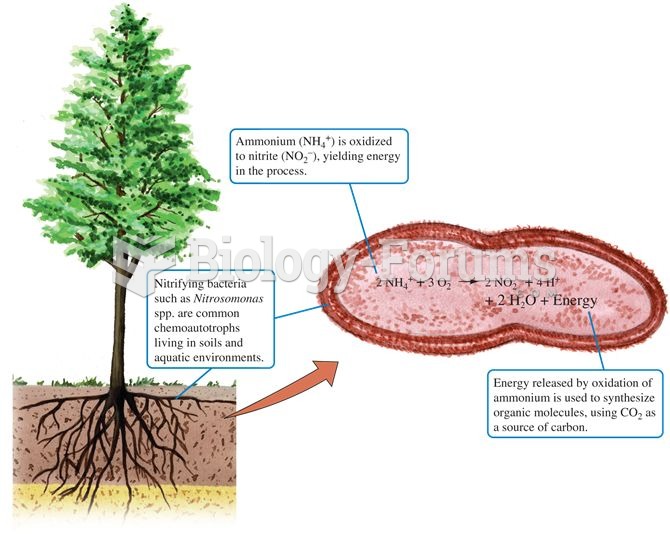
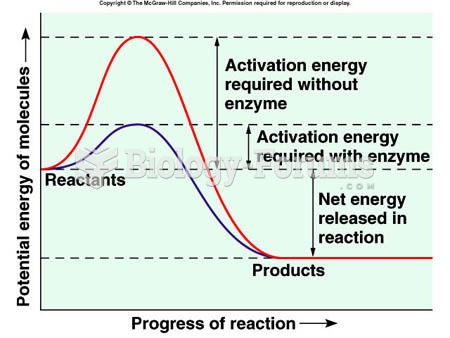
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
Did you know?
In 1835 it was discovered that a disease of silkworms known as muscardine could be transferred from one silkworm to another, and was caused by a fungus.
Did you know?
Patients who cannot swallow may receive nutrition via a parenteral route—usually, a catheter is inserted through the chest into a large vein going into the heart.
Did you know?
There are over 65,000 known species of protozoa. About 10,000 species are parasitic.
Did you know?
Cytomegalovirus affects nearly the same amount of newborns every year as Down syndrome.